Acute paracetamol poisoning
- It occurs especially in small children who have low hepatic glucuronide conjugating ability.
- maximum recommended daily dose of 80 mg/kg in children or 4 g in adults
- If a large dose (> 150 mg/kg or > 10 g in an adult) is taken, serious toxicity can occur.
- Fatality is common with > 250 mg/kg.
Clinical factors that can predispose patients to injury from acetaminophen ingestion include
- chronic alcohol ingestion,
- medications that affect the CYP2E1 enzyme system of the liver,
- malnutrition,
- genetic polymorphisms, and
- older age.
Chronic acetaminophen poisoning in the alcohol user is also characterized by markedly elevated aminotransferases (>3000 IU/L), combined with hypovolemia, jaundice, coagulopathy, hypoglycemia, and acute renal failure in greater than 50 percent of these patients.
- Early manifestations à just nausea, vomiting, abdominal pain and liver tenderness with no impairment of consciousness.
- After 12–18 hours centrilobular hepatic necrosis occurs which may be accompanied by renal tubular necrosis and hypoglycaemia that may progress to coma.
- Jaundice starts after 2 days.
Mechanism of toxicity
- N-acetyl-p-benzoquinoneimine (NAPQI) à minor metabolite of paracetamol which is detoxified by conjugation with glutathione.
- very large dose of paracetamol à glucuronidation capacity is saturated, more of the minor metabolite is formed—hepatic glutathione is depleted and
- this metabolite binds covalently to proteins in liver cells (and renal tubules) causing necrosis.
- Toxicity thus shows a threshold effect manifesting only when glutathione is depleted to a critical point
- In chronic alcoholics even 5–6 g taken in one day can result in hepatotoxicity because alcoholism induces CYP2E1 that metabolises paracetamol to NAPQI.
- Paracetamol is not recommended in premature infants (< 2 kg) for fear of hepatotoxicity.
The Drugs Controller General of India (DCGI) à (2011) issued guidelines to limit the amount of paracetamol per dosage form (single tab./cap.) to 325 mg.
Treatment
- If the patient is brought early, vomiting should be induced or gastric lavage done.
- Activated charcoal is given orally or through the tube to prevent further absorption.
- Other supportive measures, as needed, should be taken.
Specific: N-acetylcysteine 150 mg/kg should be infused i.v. over 15 min, followed by the same dose i.v. over the next 20 hours.
Alternatively, 75 mg/kg may be given orally every 4–6 hours for 2–3 days.
- While there is universal agreement that NAC is effective for the prevention of hepatic injury when administered soon after acetaminophen overdose, the exact guidelines for the initiation of treatment vary throughout the world.
- The modified Rumack-Matthew Treatment nomogram (four hour concentration of 150 mg/L) has been used for many years and is our preferred tool to guide treatment because of its safety and
- Using this approach, patients with serum acetaminophen concentrations above the line connecting 150 mcg/mL (990 micromol/L) at 4 hours and 18.8 mcg/mL (125 micromol/L) at 16 hours are considered at “possible risk” for hepatotoxicity and treatment with NAC is standard.
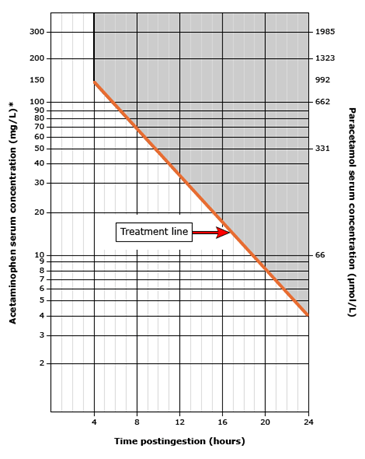
This nomogram should only be used after a single acute acetaminophen ingestion. The line indicates the level at which toxicity is possible after acetaminophen overdose. A serum acetaminophen level should be obtained four or more hours after an ingestion to ensure that a peak level has occurred. Patients who ingest extended-release preparations should have a second level drawn four hours after the first level to assess for an additional rise in serum concentration. The level should be plotted in relationship to the time of ingestion to determine the likelihood of toxicity and the need for treatment. Caution should be used in assessing the reliability of the time of ingestion. This nomogram cannot be used for ingestions that occurred greater than 24 hours prior to presentation, repeated supratherapeutic oral ingestions, or iatrogenic intravenous overdose.
Indications for N-acetylcysteine therapy include:
●Serum acetaminophen concentration drawn at four hours or more following acute ingestion of an immediate-release preparation is above the “treatment” line of the treatment nomogram for acetaminophen poisoning
●A suspected single ingestion of greater than 150 mg/kg (7.5 g total dose regardless of weight) in a patient for whom the serum acetaminophen concentration will not be available until more than eight hours from the time of the ingestion.
●Patient with an unknown time of ingestion and a serum acetaminophen concentration >10 mcg/mL
●Patient with a history of Acetaminophen ingestion and any evidence of liver injury.
●Patients with delayed presentation (>24 hours after ingestion) consisting of laboratory evidence of liver injury (ranging from mildly elevated aminotransferases to fulminant hepatic failure) and a history of excessive acetaminophen ingestion.
It replenishes the glutathione stores of liver and prevents binding of the toxic metabolite to other cellular constituents.
Ingestion-treatment interval is critical; earlier the better. It is practically ineffective if started 16 hours or more after paracetamol ingestion.
Between 10 and 20 percent of patients treated with IV N-acetylcysteine à develop an allergic or anaphylactic reactionà In the case of severe reactions à stop infusion
Approximately 33 percent of subjects treated with oral N-acetylcysteine develop nausea and vomiting. Serotonin 5-HT3 receptor antagonists (eg, ondansetron) are useful antiemetics.
