HIV
- HIV is a single stranded RNA retrovirus which uniquely carries out reverse transcription of proviral DNA from viral RNA
- The primary cell type attacked by HIV is the CD4+ helper T-lymphocyte, but later macrophages and some other cell types may also be infected.
- When population of CD4 cells declines markedly (<200 cells/microL), cell mediated immunity (CMI) is lost and opportunistic infections abound
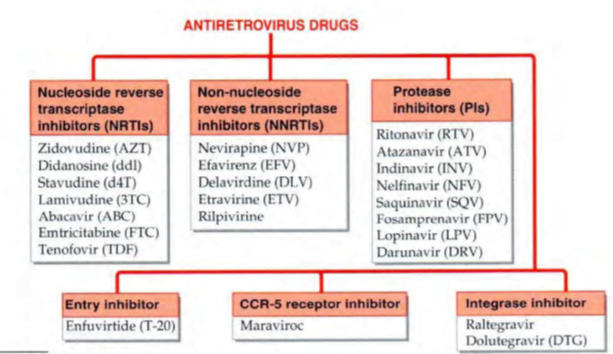
Nucleoside reverse transcriptase inhibitors (NRTls)
Zidovudine
- Zidovudine itself gets incorporated into the proviral D A and terminates chain elongation.
- prevents infection of new cells by HIV, but has no effect on proviral DNA that has already integrated into the host chromosome.
- Resistance to AZT occurs by point mutations which alter reverse transcriptase enzyme.
- Adverse effects à Anaemia and neutropenia are the most important and dose-related adverse effects.
- Myopathy, pigmentation of nails, lactic acidosis, hepatomegaly are infrequent.
- Interactions Paracetamol increases AZT toxicity, probably by competing for glucuronidation. Azole antifungals also inhibit AZT metabolism.
- Stavudine and zidovudine exhibit mutual antagonism by competing for the same activation pathway
- it is no longer included in the preferred I st line WHO (2016) regimen, but is a component of one alternative regimen
Didanosine (ddl)
- In contrast to AZT, it does not cause myelosuppression.
- The major dose-related toxicity is peripheral (stocking and glove) neuropathy, which may be irreversible.
- Rarely acute pancreatitis occurs.
Stavudine (d4T)
- Because of long term serious metabolic complications like lipodystrophy, lactic acidosis and peripheral neuropathy stavudine is no longer employed in WHO as well as NACO regimens. except in exceptional circumstances.
Lamivudine (3TC)
- Point mutation in HIV-reverse transcriptase and HBV-DNA polymerase gives rise to rapid lamivudine resistance
- Lamivudine is also effective in chronic hepatitis B; in which lower doses are required than for HIV. This may be due to longer intracellular t½ of lamivudine in HBV infected cells than in HIV infected cells.
- HBV-DNA titre is markedly reduced and biochemical as well as histological indices of liver function improve.
- However, viral titres rise again after discontinuation. Even with continued medication HBV viraemia tends to return and lamivudine-resistance develops in upto 70% patients within 1- 5 years.
- Because of this, it is no longer a I st line drug for chronic hepatitis B.
Abacavir (ABC)
- Hypersensitivity reactions such as rashes, fever, abdominal pain, bowel upset, flu-like respiratory and constitutional symptoms occur in 2- 5% adult patients and are the major problems.
- Abacavir must be promptly stopped when the reaction occurs à genetic basis and massive release of TNFa have been related to this reaction.
- Abacavir should never be given again to a patient who has developed this reaction.
Tenofovir (TDF)
- only nucleotide (not nucleoside) analogue that is a commonly used anti- HIV drug.
- also active against HBV
Emtricitabine (FTC)
- Like lamivudine, it is also active against HBV, but should not be used in HIV-HBV co-infected patients because sudden stoppage of therapy can cause rebound exacerbation of hepatitis
Non-nucleoside reverse transcriptase inhibitors (NNRTls) à Nevirapine (NVP), Efavirenz (EFV)
- inhibit HIV reverse transcriptase without the need for intracellular phosphorylation
- they are non-competitive inhibitors.
- They are more potent than AZT on HIV-1 , but do not inhibit HIV-2.
- Cross-resistance between NVP and EFV is common, but not with RTls or PIs.
- A patient failing a regimen containing NVP should not be treated with EFV and vice versa, but can be put on etravirine regimen.
Etravirine
- second generation NNRTI which is active against HIV-1 mutants that are resistant to other NNRTls.
- Etravirine is approved for use only in combination with a NRTI + one of PIs,
Retroviral protease inhibitors (Pis)
- Because they act at a late step of viral cycle, they are effective in both newly as well as chronically infected cells.
- All PIs (especially ritonavir and lopinavir) are potent inhibitors of CYP3A4
- Pls are avoided in 1st line regimens, because their use in initial regimens markedly restricts second line regimen options. Most guidelines, including that of WHO and NACO, reserve them for failure cases.
- In case of different Pls, 6-18 tablets are to be taken daily, some on empty stomach, but others with meals; and this has to go on for months and yearsà patient acceptability and compliance are often low.
- One of the strategies adopted to reduce the dose of PIs (except that of NFV) is to combine them with a low and subtherapeutic dose ( I 00 mg) of ritonavir à reducing first pass metabolism, ritonavir increases the bioavailability and by slowing systemic metabolism decreases clearance of the companion Pl.
- ‘boosted Pl regimen’ permits reduction in the number/frequency of tablets to be taken each day.
- Nelfinavir is not to be combined with ritonavir because it is metabolized mainly by CYP2Cl9 that is not inhibited.
- The most prominent adverse effects à gastrointestinal intolerance
- Other s/e à lipodystrophy (abdominal obesity, buffalo hump with wasting of limbs and face), dyslipidaemia
- Indinavir crystalizes in urine à increases risk of urinary calculi.
- Darunavir is approved for use only in combination with ritonavir or another booster Cobicistat.
lntegrase inhibitors
- The HIV integrase enzyme enters the host cell along with the genomic RNA.
- After the HIV proviral DNA is transcripted in the cytoplasm of host cell , this enzyme trans locates to the nucleus along with the proviral DNA, nicks host chromosomal DNA, integrates the proviral DNA with it and reseals it. Thus the proviral DNA becomes a part of the chromosomal DNA, and the cell gets permanently infected
- Interference with this virus specific function à lntegrase inhibitors
- Raltegravir – active against both HIV- I and HI V-2, and there is no cross resistance with other classes of ARV drugs
- Administration of Ca2+ and Mg+ containing antacids and Ca2+ or iron supplement should be avoided with raltegravir or staggered from it, because of its chelating property.
- Dolutegravir à second generation integrase inhibitor
Entry (fusion) inhibitor à Enfuvirtide
- binding to HIV-I envelope transmembrane glycoprotein (gp4 I) which is involved in fusion of viral and cellular membranes.
- Not active against H IV-2.
- No cross resistance with other classes of ARV drugs occurs.
- Administered s.c. twice daily, it is used as add-on drug to an optimized regimen in selected patients who have failed many earlier regimens or have multidrug resistant HI V.
- The injections are painful and cause local nodules/cysts.
CCRS receptor inhibitor à Maraviroc
- The globular glycoprotein gp 120 of the HIV envelope anchors to the CD4 site of host cell by binding to a cell membrane receptor, which mostly is the CCRS chemokine receptor (most HIV arc CCR5-tropic).
- Maraviroc à targets the host cell CCRS receptor and blocks it.
- Attachment of the virus and subsequent entry of viral genome into the cell is thus interfered.
- It has no effect on HIV strains that are CXCR4 receptor tropic
- has resulted in marked reduction in HIV-RNA load. and improvement in CD4 count.
- active orally and there is no cross resistance with any other ARV drug.
HIV TREATMENT PRINCIPLES AND GUIDELINES
- ‘ highly active antiretroviral therapy’ (HAART) with combination of 3 or more drugs whenever indicated.
- Monotherapy is contraindicated.
- It has been realized that even with 3 drug ART, which rapidly kills > 99% virions, a small number survive within the resting CD4 lymphocytes and invariably give rise to relapse when treatment is discontinued despite complete absence of detectable viraemia and nomral CD4 cell count for years.
- Relapses occur even if the same A RT is continued after disappearance of viraemia and immune reconstitution à because HIV-reverse transcriptase and other enzymes are highly copying error prone, implying that viral replication produces changes at some base pairs (and codons) with high frequency-rate of mutation is high.
- early institution of ART is associated with better clinical outcomes in people living with HIV (PL-HIV) compared to late start ART
- early-start ART results in a smaller pool of chronically virus infected CD4 cells and lower chances of drug resistance.
- The WHO (2016) guideline bas recommended that ART should be started in all adults including pregnant and breast-feeding women, adolescents as well as children as soon as possible after diagnosis or HIV infection is confirmed, irrespective of the CD4 cell count or the HIV-RNA load or the clinical stage of the disease.
- The WHO recommendation of immediate-start ART in all HIV positive subjects has been accepted by the Govt. of India, and is being implemented by NACO
Therapeutic regimens
- Whenever ART is instituted, it should be aggressive with at least 3 anti-HIV drugs.
- optimum response to any regimen
- reduction of plasma HIV-RNA to undetectable levels (<50 copies/μL) and restoration of CD4 cell count to near normal within 6 months.
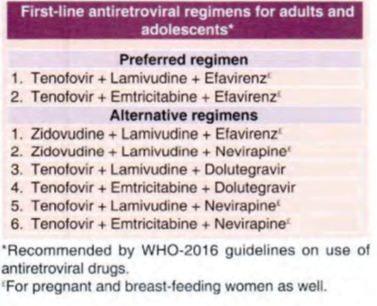
First line regimens universally include 2 NRT!s + I NNRTl.
Placing emphasis over efficacy and tolerability, the preferred NRTI s are lamivudine, abacavir, tenofovir and emtricitabine.
Efavirenz is preferred over nevirapine as the NNRTI,
important general points are:
- The 3 drugs in the regimen should belong to at least 2 different classes. Single class regimens are inferior.
- For treatment-naive patients, only Pl sparing regimens (2 N RTI + NNRTI/Integrase inhibitor) are chosen. They are more convenient with lower pill burden, simpler dosing schedules, more acceptable
- In patients with metabolic disorders, dolutegravir/raltegravir containing regimens should be preferred over Pl containing regimens.
- The Pl containing regime ns (2 NRTI + PI or NRTI + NNRTI + Pl) are reserved for advanced cases who have failed earlier regimens.
- Treatment is life-long.
- Institution of ART in patients with latent or partially treated opportunistic infection à may produce ‘immune reconstitution inflammatory syndrome’ (IRIS) characterized by marked inflammatory reaction against residual organisms and constitutional symptoms due to ‘ reestablishment’ of immune function.
- Pregnancy in women does not contraindicate ART. à relatively safe à zidovudine, lamivudine, tenofovir, emtricitabine, efavirenz, nevirapine and nelfinavir.
- Therapy should not be discontinued during an acute opportunistic infection, except in case of intolerance, interactions or toxicity.

Changing a failing regimen
An ART regimen is considered to have failed when:
• Plasma HIV-RNA count is not rendered undetectable (<50 copies/~1L) within 6 months therapy.
• Repeated detection of virus in the plasma after initial suppression to undetectable levels despite continuation of the drug regimen.
• Clinical deterioration, fall in CD4 cell count, serious opportunistic infection while continuing drug therapy.
- The failed regimen should be changed entirely (all 3 drugs changed) to drugs that have not been administered earlier.
- However, lamivudine, even if administered in the failed regimen, may be continued in the second-line regimen, because it may exert residual antiviral activity and has the potential to reduce viral fitness, as well as improve viral sensitivity to AZT and TDF.
- A boosted Pl is nearly always included in 2nd line regimens.
- The WHO (20 16) guidelines recommend: àThe 2nd line ART regimens should consist of two NRTls + one ritonavir boosted Pl.
- The preferred ritonavir boosted Pl options for 2nd line regimens are FDCs of atazanavir or lopinavir.

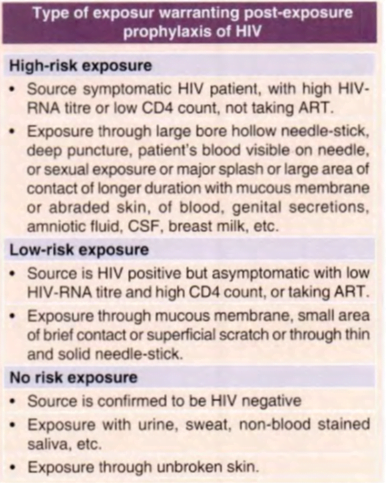

Salient Points of NACO-October 2018 Guidelines
| When to start ART: | In all patients irrespective of CD4 count or symptoms |
| 2. First Line ART: | |
| a) Adults and adolescents | T+ L +E |
| b) Children | A+ L+E |
| c) Any person with weight less than 30Kg | A+ L+E |
| d) If elevated serum creatinine | A+ L+E |
| e) Infection with HIV-2 (or HIV-1 + HIV-2) | T+ L +L/r |
| 3. ART in pregnancy and breastfeeding | |
| a) ART-native female | T+ L +E |
| b) Already on ART | Continue same |
| c) Exposure too nevirapine alone in prior pregnancy | T+ L +L/r |
| 4. Infant prophylaxis: | |
| a) Mother taking ART for ≥ 4 weeks | N for 6 weeks |
| b) Mother taken ART for < 4 weeks | N for 12 weeks |
| c) Mother exposed to nevirapine previously | Z for 6/12 weeks |
| 5. Post-exposure Prophylaxis: | T+ L +L/r |
Abbreviations used:
T: Tenofovir L: Lamivudine E: Efavirenz A: Abacavir L/r: Lopinavir + Ritonavir N: Nevirapine
The first dose of Post exposure prophylaxis should be administered ideally within 2 hours (but certainly within the first 72 hours) of exposure and the risk evaluated as soon as possible
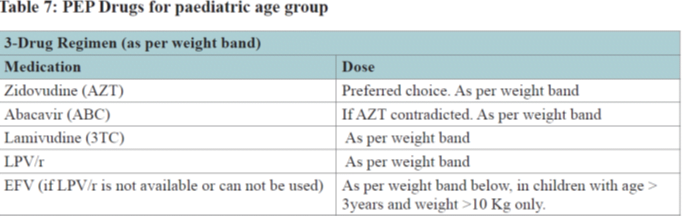
Recent advances in HIV
New NRTI
APRICITABINE – March 2011
- Cytidine analogue & active against HIV-1
- Effective in patients refractory to treatment to lamivudine or emtricitabine containing regimen
- Promising against NRTIs-resistant HIV strains
- In March 2011, FDA agreed for shorter, single Phase III trial design (300 patients dosed for 2 weeks) before approval
ELVUCITABINE
- Cytidine analogue, active against HIV-1 (Phase II clinical trials)
- A phase II trial of 48 weeks showed similar efficacy & safety profile in treatment-naïve patients as compared to 3TC (lamivudine)
- Chemical structure similar to lamivudine &emtricitabine
- In vitrostudies suggested, action on certain NRTIs resistant HIV strains (lamivudine and emtricitabine) & anti-HBV activity
AMDOXOVIR
- Designed for use against first line NRTI resistant HIV mutants
- withdrawn prior to study enrollment due to safety issues à major adverse effects: obstructive nephropathy, lens opacities
Newer NNRTI
Doravirine – August 2018
specifically indicated for use in combination with other antiretroviral agents for the treatment of HIV-1 infection in adult patients with no prior antiretroviral treatment history.
- More favorable CNS, Lipid and SGOT/SGPT profile compared with Efavirenze
- Tablet 100mg
- Dizziness, nightmares, depression
ETRAVIRINE – Jan 2008
- Second generation NNRTIs
- Resistance to other NNRTIs do not confer resistance to etravirine
- Jan 2008, FDA approved its use for patients with established resistance to other ARVs
- Indicated for treatment of HIV-1 infection in ART-experienced adult patients
RILPIVIRINE
- In august-2011, U.S. FDA approved FDC of rilpivirine with emtricitabine& tenofovir.
- Higher potency, longer half-life & reduced side-effect profile compared with older NNRTIs
- In combination with emtricitabine& tenofovir, shown to have higher rates of virologic failure in patients with baseline HIV viral loads greater than 100,000 copies/mm3
- High barrier to drug resistance
- Effective against HIV strains resistant to conventional NNRTIs
Integrase inhibitor à DOLUTEGRAVIR — August 2013
Used to treat HIV-infected adults:
•Including those who have been treated with other integrase strand transfer inhibitors
•Treatment naïve & experienced HIV patients
•Approved: children aged 12 years &
weighing at least 40 kg (treatment-naïve /experienced)
•S/E: insomnia and headache, allergic reactions & abnormal LFT
ELVITEGRAVIR à August, 2012
For use in adult patients starting HIV Rx for first time as part of FDC known as Stribild(Elvitegravir/cobicistat/emtricitabine/tenofovir)
•FDA approved Elvitegravir as single pill formulation (September 24, 2014)
•Used for treatment of HIV-1 infection in adults (treatment experienced pt.)
•Must be used in combination with a protease inhibitor that is co-administered with ritonavir
•S/E-Diarrhoea
CABOTEGRAVIR
Investigational new drug under phase III clinical trial
•Conferring an exceptionally long half-lifeof 21–50 days following a single dose
•Making possible suppression of HIV with dosing as infrequently as once every three months
ENTRY/ FUSION INHIBITORS
VICRIVIROC
Noncompetitive allosteric antagonist of CCR5
•OD oral dosing can be done as it is effective at nanomolar concentrations
•Induces a conformational change of extracellular segment of CCR5
•Prevents binding of gp120 to target CD4 cell, consequently preventing the virus from entering the target cell at all
CD4 RECEPTOR ATTACHMENT INHIBITORS à IBALIZUMAB – March 2018
Specific Treatments:
multidrug resistant HIV-1 infection
Humanized monoclonal IgG4 antibody against CD4 receptors
•Inhibits viral entry by attaching with extracellular domain of CD4 receptors
•Biding site is distinct from gp120 &is designed in a way so as not to interfere with immunological function of CD4 receptors
•Cross-resistance with other ARVs is rare due to novel mechanism
COBICISTAT – (FDA-sept. 2013)
No anti-HIV activity but is a potent inhibitor of CYP3A
•Increases blood level of certain ARV drugs, allowing once-daily dosing
•Safe & well-rotated in escalating single & multiple dose-ranging studies in healthy volunteers
•Boosting agent for PIs, especially antazanvir& integraseinhibitor elvitegravir(FDC found to be comparable with ritonavir)
Stribild (Elvitegravir/cobicistat/emtricitabine/tenofovir)
- Approved as FDC with
- Elvitegravir/cobicistat/emtricitabine/tenofovir alafenamide
- Darunavir/cobicistat
- Atazanavir/cobicistat.
ZINC FINGER INHIBITORS
- Inner core of HIV is called nucleocapsid held by structures called “zinc fingers”.
- Zinc finger inhibitors (or zinc ejectors) are drugs that can break apart these structures and prevent the virus from functioning
- Scientists believe that the nucleocapsid core cannot mutate very easily, so a drug that works against zinc fingers might be effective for a long time.
- Drugs that attack them could have serious side effects.
- One zinc finger inhibitor – azo dicarbonamide (ADA) – has been tested in a Phase I/II trial
FDC
- bictegravir/emtricitabine/tenofovir alafenamide – February 2018
- once daily single tablet regimen combining the novel, unboosted integrase strand transfer inhibitor (INSTI) bictegravir, with
- the demonstrated safety and efficacy profile of the Descovy (FTC/TAF) dual nucleoside reverse transcriptase inhibitor (NRTI) backbone.
- specifically indicated as a complete regimen for the treatment of HIV-1 infection in adults who have no antiretroviral treatment history or to replace the current antiretroviral regimen in those who are virologically suppressed (HIV-1 RNA <50 c/mL) on a stable antiretroviral regimen for at least three months with no history of treatment failure
- doravirine, lamivudine, and tenofovir disoproxil fumarate – Aug 2018
three-drug combination of doravirine (a nonnucleoside reverse transcriptase inhibitor [NNRTI]), lamivudine, and tenofovir disoproxil fumarate (both nucleoside analogue reverse transcriptase inhibitors).
specifically indicated as a complete regimen for the treatment of HIV-1 infection in adult patients with no prior antiretroviral treatment history.
- darunavir, cobicistat, emtricitabine, and tenofovir alafenamide – July 2018 – for HIV 1 infection
