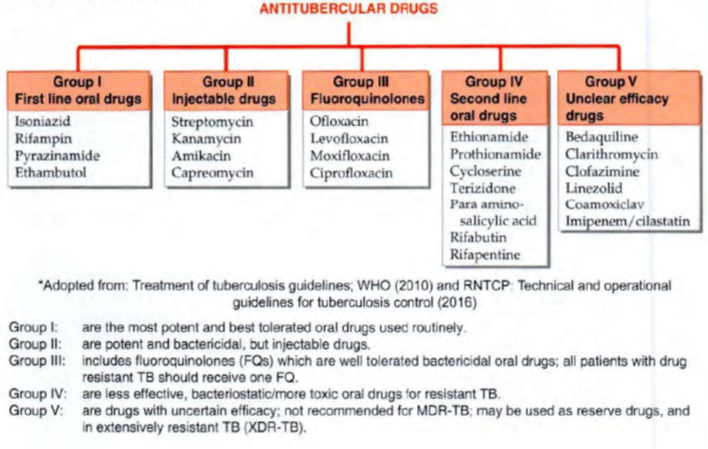
Antitubercular Drugs
- chronic granulomatous disease and
- a major health problem in developing countries.
- About 1/3rd of the world’s population is infected with Mycobact. Tuberculosis
- WHO à2014, 9.46 million active TB cases globally,
- India à highest contributor with 2.2 million cases
- 600 people die from TB every day.
- In 2012, the Government of India à declared TB à notifiable disease, so that any doctor who treats a TB patient, has to notify it to the Govt.
- In India, control and treatment of TB is covered under a National programme which provides free treatment to all TB cases
- RNTCP – last revised 2016
- India à 2.1 million à HIV in 2015 à have 10 % risk of developing TB
- As per latest survey, in India ~ 3% of new cases and 12- 17% of previously treated patients have MDR-TB.
Isoniazid (Isonicotinic acid hydrazide)
- an essential component of all antitubercular regimens, unless the patient is not able to tolerate it or bacilli are resistant.
- primarily tuberculocidal
- primarily tuberculocidal.
- Fast multiplying organisms are rapidly killed, but quiescent ones are only inhibited.
- acts on extracellular as well as on intracellular TB (bacilli present within macrophages), and
- equally active in acidic or alkaline medium.
- mechanism of action à inhibition of synthesis of mycolic acids which are unique fatty acid components of mycobacterial cell wallà explain the high selectivity of INH for mycobacteria (it is not active against any other microorganism).
- INH enters sensitive mycobacteria which convert it by a catalase-peroxidase enzyme into a reactive metabolite à inhibits InhA and KasA.
- The reactive INH metabolite à forms adduct with NADP as well which inhibits mycobacterial DHFRase à interruption of DNA synthesis.
- About 1 in 106 tubercle bacilli is inherently resistant to clinically attained INH concentrations.
- If INH is given alone, such bacilli proliferate selectively and after 2–3 months (sometimes even earlier) an apparently resistant infection emerges.
- Mechanism à INH resistance à mutation of the catalase-peroxidase (KatG) gene so that the bacilli do not generate the reactive metabolite of INH.
- INH resistance may also involve mutation in the inhA or kasA genes.
- Resistance based on efflux of INH from the bacterial cell is also possible.
- According to WHO, the global weighted mean of any INH resistance (excluding MDR) among new TB patients is 7.4%. In India resistance to INH alone or in combination with other anti-TB drugs is estimated to be 18%.
- Combined with other drugs, INH has good resistance preventing action. No cross resistance with other antitubercular drugs occurs.
- Pharmacokinetics à completely absorbed orally and penetrates all body tissues, tubercular cavities, placenta and meninges.
- Extensively metabolized in liver à acetylation
- The rate of INH acetylation shows genetic variation
- Fast acetylators (30–40% of Indians) t½ of INH 1 hr.
- Slow acetylators (60–70% of Indians) t½ of INH 3 hr.
- acetylator status does not matter if INH is taken daily, but biweekly regimens are less effective
- in fast acetylators.
- inhibiting CYP2C19 and CYP3A4, à retards phenytoin, carbamazepine, diazepam, theophylline and warfarin metabolism à may raise their blood levels.
- Since rifampin is an enzyme inducer, its concurrent use counteracts the inhibitory effect of INH.
Adverse effects
- Peripheral neuritis and a variety of neurological manifestations (paresthesias, numbness, mental disturbances, rarely convulsions) à dose-dependent toxic effects. à due to interference with production of the active coenzyme pyridoxal phosphate from pyridoxine, and its increased excretion in urine
- Pyridoxine given prophylactically (10 mg/day) prevents the neurotoxicity even with higher doses.
- Prophylactic pyridoxine must be given to diabetics, chronic alcoholics, malnourished, pregnant, lactating and
- HIV infected patients, but routine use is not mandatory.
- INH neurotoxicity à t/t à pyridoxine 100 mg/day.
- Hepatitisà more common in older people and in alcoholics (chronic alcoholism induces CYP2E1 which generates the hepatotoxic metabolite).
- Other side effects are lethargy, rashes, fever, acne and arthralgia.
Rifampin (Rifampicin, R)
- bactericidal
- as efficacious as INH
- acts best on slowly or intermittently dividing ones (spurters).
- M. leprae is highly sensitive, while MAC and some other mycobacteria, but not M. fortuitum, are moderately susceptible.
- Both extra- and intracellular organisms are affected.
- MOA à interrupts RNA synthesis by binding to β subunit of mycobacterial DNA-dependent RNA polymerase (encoded by rpoB gene) and blocking its polymerizing function.
- rifampin resistant à In India it is estimated to be 2%.
- nearly always due to mutation in the rpoB gene reducing its affinity for the drug.
- No cross resistance with any other antitubercular drug, except rifampin congeners, has been noted.
Pharmacokinetics
- well absorbed orally, (bioavailability is ~ 70%),
- but food decreases absorption à to be taken in empty stomach.
- Though it crosses meninges, it is largely pumped out from CNS by P-glycoprotein.
- metabolized in liver
- The t½ of rifampin is variable (2–5 hours).
- Microsomal enzyme inducer—
- Enhances its own metabolism
- as well as that of many drugs including warfarin, oral contraceptives, corticosteroids, sulfonylureas,
- steroids, HIV protease inhibitors, nonnucleoside reverse transcriptase inhibitors (NNRTIs), theophylline, metoprolol, fluconazole, ketoconazole, clarithromycin, phenytoin, etc.
- Contraceptive failures have occurred.
- Adverse effects
- Hepatitis, a major adverse effect, generally occurs in patients with preexisting liver disease àdose-related
- Development of jaundice requires discontinuation of the drug—then it is reversible.
- Minor reactions à Cutaneous syndrome: flushing, pruritus + rash (especially on face and scalp), redness
- and watering of eyes.
- Flu syndrome: with chills, fever, headache, malaise and bone pain.
- Abdominal syndrome: nausea, vomiting, abdominal cramps with or without diarrhoea.
- Urine and secretions may become orange-red—but this is harmless.
- Other serious but rare reactions are:
- Respiratory syndrome: breathlessness which may be associated with shock and collapse.
- Purpura, haemolysis, shock and renal failure.
- Other uses of rifampin
- 1. Leprosy
- 2. Prophylaxis of Meningococcal and H. influenzae meningitis and carrier state.
- 3. Second/third choice drug for MRSA, diphtheroids and Legionella infections.
- 4. Combination of doxycycline and rifampin is the first line therapy of brucellosis.
Pyrazinamide (Z)
- Weakly tuberculocidal
- more active in acidic medium.
- more lethal to intracellularly located bacilli and to those at sites showing an inflammatory response (pH is acidic at both these locations).
- highly effective during the first 2 months of therapy when inflammatory changes are present.
- By killing the residual intracellular bacilli it has good ‘sterilizing’ activity.
- Its inclusion has enabled duration of treatment to be shortened and risk of relapse to be reduced.
- The mechanism of action à converted inside the mycobacterial cell into an active metabolite pyrazinoic acid by an enzyme (pyrazinamidase) encoded by the pncA gene.
- This metabolite gets accumulated in acidic medium and probably inhibits mycolic acid synthesis, but by interacting with a different fatty acid synthase.
- Resistance à mostly due to mutation in the pncA gene.
- has good penetration in CSF, because of which it is highly useful in meningeal TB;
- Hepatotoxicity à most important dose related adverse effect
- contraindicated in patients with liver disease.
- Hyperuricaemia is common and is due to inhibition of uric acid secretion in kidney: gout can occur.
- Other adverse effects à abdominal distress, arthralgia, flushing, rashes, fever and loss of diabetes control: repeated blood glucose monitoring is warranted in diabetics.
Ethambutol (E)
- selectively tuberculostatic
- Fast multiplying bacilli are more susceptible.
- Added to the triple drug regimen of RHZ à hasten the rate of sputum conversion and to prevent development of resistance
- mechanism of action à inhibit arabinosyl transferases (encoded by embAB genes) involved in arabinogalactan synthesis à interfering with mycolic acid incorporation in cell wall.
- Resistance à mutation in embB gene
- No cross resistance with any other antitubercular drug
- Patient acceptability à very good and side effects are few.
- Loss of visual acuity/colour vision, field defects due to optic neuritis is the most important dose and duration of therapy dependent toxicity.
- Patients should be instructed to stop the drug at the first indication of visual impairment.
- Because young children may be unable to report early visual impairment, it was contraindicated, but is now allowed with due precaution.
- Hyperuricemia à interference with urate excretion. It is safe during pregnancy.
Streptomycin (S)
- tuberculocidal, but less effective than INH or rifampin;
- acts only on extracellular bacilli (because of poor penetration into cells).
- penetrates tubercular cavities, but does not cross to the CSF, and has poor action in acidic medium.
- In case of S-resistant infection, it must be stopped at the earliest because of risk of S-dependence, in which case the infection flourishes when the drug is continued.
- Because of need for i.m. injections and lower margin of safety (ototoxicity and nephrotoxicity, especially in the elderly and in those with impaired renal function) S is used only as an alternative to or in addition to other 1st line anti-TB drugs.
- Use is restricted to a maximum of 2 months. à ‘supplemental’ 1st line drug.
SECOND LINE ANTI-TB DRUGS
- used only in case the bacilli are resistant to one or more 1st line drugs or when these are not tolerated/are contraindicated.
- The RNTCP standardized regimen for MDR-TB includes Km (probably because it is less expensive than Am), but in many countries Am is preferred, because it is considered less toxic.
- Cross resistance between Km and Am is very common.
- Both Km and Am produce less vestibular toxicity than hearing loss, but are equally nephrotoxic.
- Patients should be instructed to report vertigo and tinnitus.
- Capreomycin (Cm) à similar mycobactericidal activity, ototoxicity and nephrotoxicity
- It has to be injected i.m. and is used only as alternative to aminoglycoside antibiotics.
- Many M.tuberculosis isolates resistant to S and Am, as well as MDR-TB remain susceptible to Cm.
Fluoroquinolones (FQs)
- Fluoroquinolones (FQs) like ofloxacin (Ofx), levofloxacin (Lfx), ciprofloxacin (Cfx) and moxifloxacin (Mfx) are relatively new potent oral bactericidal drugs for TB
- Mfx is the most active FQ against M.tuberculosis
- Cfx is more active than Lfx against atypical mycobacteria.
- The FQspenetrate cells and kill mycobacteria lodged inside macrophages as well.
- Cfx à not favoured à because of its extensive use in other bacterial infections and chances of resistance.
- primary indication of FQs à treatment of drug resistant TB.
The RNTCP 2016 have included Lfx in the standardized regimen for MDR-TB.
Ethionamide (Eto)
- Chemically it resembles INH, but contains sulfur.
- The mechanism of action is also similar to INH: it is converted by mycobacteria into an active intermediate which interferes with mycolic acid synthesis.
- Resistance à mutation of the gene that encodes for the Eto activating enzyme.
- Pyridoxine (100 mg/day) can mitigate the neurological adverse effects
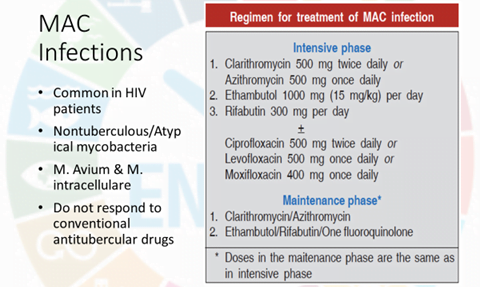
- Ethionamide is used only for drug-resistant TB. It is a component of the RNTCP standardized regimen for MDR-TB and an optional drug for inclusion into the treatment regimen of MAC infection in AIDS patients.
- It is also a reserve drug for leprosy.
Cycloserine (Cs)
- inhibits bacterial cell well synthesis by inactivating the enzymes which racemize L-alanine and link
two D-alanine residues.
- Cs is tuberculostatic
- Cs is contraindicated in patients with a history of mental illness or seizures.
- Cycloserine is used only for resistant TB, especially MDR cases. It is included in the standardized regimen used by RNTCP for MDR-TB.
Para-amino salicylic acid (PAS)
- MOA à inhibition of folate synthase.
- PAS is tuberculostatic and one of the least active drugs
- PAS is used only in resistant TB. The RNTCP includes it in the standardized regimen for MDR-TB only when one of the tuberculocidal drugs (Km, Ofx, Z, Eto) or both the static drugs (E, Cs) cannot be used.
Bedaquiline (BDQ)
- inhibits mycobacterial ATP synthase, thereby limiting energy production within mycobacterial
cell.
- The human ATP synthase is 20,000 fold less sensitive to BDQ than is the mycobacterial enzyme.
- exhibited strong bactericidal and sterilizing activity against M. tuberculosis in in vitro tests and in animal models by killing both rapidly multiplying as well as dormant bacilli.
- The MICs of BDQ are very low and similar for drug sensitive as well as for MDR-TB and XDR-TB s trains.
- Many nontubercular mycobacteria and M. leprae are also inhibited in vitro.
- resistance à primarily by mutation of ATP synthase enzyme, reducing its affinity for BDQ. Also, acquisition of BDQ efflux from the bacilli.
- However, no cross resistance between BDQ and any 1st line or 2nd line anti-TB drug occurs
- fatty meal improves absorption.
- Metabolism occurs in liver, mainly by CYP3A4,
- Terminal t½ of BDQ is very long (- 160 days). probably due to redistribution from tissues. It is excreted mainly in faeces.
- In clinical trials on pulmonary MDR-TB patients, addition of BDQ to WHO regimen for MDR-TB reduced
the time for sputum to become culture negative.
- However, in 2 clinical trials, despite better microbiological outcome, more patients died in the BDQ group than in those receiving only background regimen.
- Since all deaths occurred after BDQ was stopped, the deaths did not appear to be related 10 BDQ use.
- The US-FDA approved BDQ in 201 2, and it has been marketed internationally as SIRTURO 100 mg tab.
- The WHO in 2013 have issued guidelines for use of BDQ in drug resistant TB, following which the RNTCP (2016) has introduced BDQ in India for MDR-TB at selected centers through its ‘conditional access programme’.
The present recommendations regarding use of BDQ are:
• It should be used only for pulmonary MDR-TB in adults (> 18 yr)
• Women patients should be nonpregnant and willing to remain so during BDQ use.
• It should be used only in combination with at least 3 other anti-TB drugs to which the bacilli of the
patient are shown to be susceptible in vitro. or at least 4 other drugs to which the patient’s isolate is likely
to be sensitive
• It should be used only when an effective regimen cannot otherwise be provided.
• BDQ should be given for a maximum of 24 weeks in a dose or 400 mg/day for 2 weeks followed by 200
mg 3 times a week for the next 22 weeks.
• Each BDQ tablet should be Swallowed whole with meals.
• The background anti-TB drugs should be continued after stopping BDQ for the total 24 months treatment of MDR-TB. This is to ensure that any surviving bacilli arc not exposed to BDQ alone, which persists in the body for > 5 months after stopping.
• BDQ is not to be used for drug-sensitive TB, or extrapulmonary TB or for nontubercular mycobactcria.
Adverse effects à nausea. headache, arthralgia and prolongation of QTc interval. Caution is required in
using BDQ in patients taking other QTc prolonging drugs. BDQ has the potential to cause hepatotoxicity.
TREATMENT OF TUBERCULOSIS
(a) Rapidly growing with high bacillary load à in the wall of a cavitary lesion where oxygen tension is high
and pH is neutral. These bacilli are highly susceptible to H and to a lesser extent to R, E and S.
(b) Slow growing à intracellularly (inside macrophages) à particularly vulnerable to Z, while H, R and E are less
active. and S is inactive.
(c) Spurters à within caseous material where oxygen tension is low à R is most active on this subpopulation.
(d) Dormant à bacilli remain totally inactive for prolonged periods. No antitubercular drug (except probably
bedaquiline) is significantly active against them.
The goals of antitubercular chemotherapy are:
(a) Kill dividing bacilli à Drugs with early bactericidal action à rapidly reduce bacillary load à makes the patient non-contagious
(b) Kill persisting bacilli à sterilize the patient and prevent relapse.
(c) Prevent emergence of resistance
The general principles of antitubercular chemotherapy are:
- Use of any single drug in tuberculosis results in the emergence of resistant organisms and relapse in almost 3/4th patients.
- A combination of two or more drugs must be used.
rationale is:
- the incidence of resistant bacilli to most drugs ranges from 10–8 to 10–6.
- Because an average patient of pulmonary tuberculosis harbours 108 to 1010 bacilli, the number of organisms that will not respond to a single drug is high and cannot be dealt by the host defence.
- During protracted treatment, these bacilli multiply and become dominant in 3–4 months.
- Because insensitivity to one drug is independent of that to another, i.e. incidence of H resistance among bacilli resistant to R will be 10–6 and vice versa; only few bacilli will be resistant to both; these can be handled by host defence.
Isoniazid and R are the most efficacious drugs; their combination is definitely synergistic—duration of therapy is shortened from > 12 months to 9 months. Addition of Z for the initial 2 months further reduces duration of treatment to 6 months.
- Response is fast in the first few weeks as the fast dividing bacilli are eliminated rapidly.
- Symptomatic relief is evident within 2–4 weeks.
- The rate of bacteriological, radiological and clinical improvement declines subsequently as the slow multiplying organisms respond gradually.
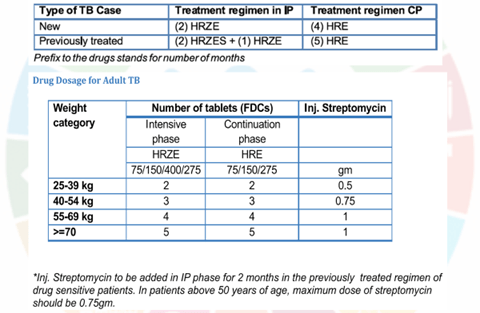
For devising drug regimens, the RNTCP (2016) has classified TB cases into:
- Drug-sensitive TB:
The patient’s bacilli are susceptible to all 5 first line anti-TB drugs (ATDs).
All new TB patients who have never taken any ATD or have taken them for less than one month
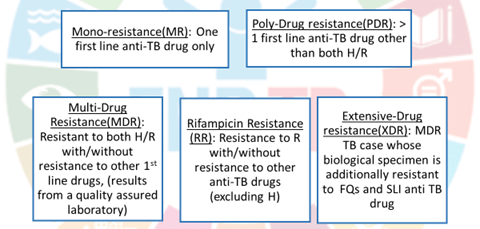
2. Multidrug resistant TB (MDR-TB):
resistant to both R and H with or without resistance to any number of other I st line drugs.
3. Rifampin resistant TB (RR-TB):
resistant to R but not to H, with or without resistance to other ATDs. These patients are to be treated as if they have MDR-TB.
4. Mono-resistant TB:
Resistant to one 1st line ATD, but not R-resistant.
5. Poly-drug resistant TB (PDR-TB):
resistant to more than one 1st line ATD, except both R and H resistance.
6. Extensive drug resistant TB (XDR-TB):
MDR-TB case whose bacilli are additionally resistant to a FQ and one 2nd line injectable ATD.
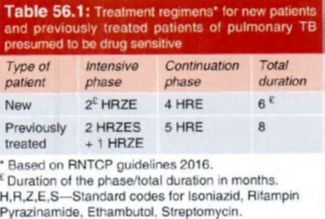

- While in the 20 10 RNTCP regimen, the continuation phase had only 2 drugs (RH), the 2016 regimen includes E as well.
- This is in consonance with the suggestion of WHO to include E in the continuation phase for areas with high incidence of primary H resistance.
- In the new guidelines, the frequency of dosing during both IP and CP has been changed from 3 times a week to daily administration, which is considered optimal.
- The thrice weekly dosing was a compromise in the face of operational constraints of the programme, which have now been overcome.
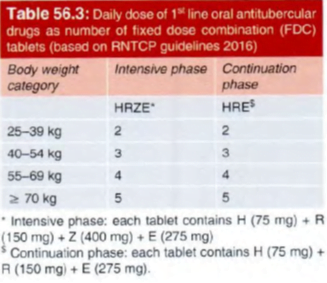
- it is advisable to use fixed dose combinations (FDCs) of oral ATDs, because this ensures that the patient takes all the drugs; risk of bacilli being exposed to only I or 2 ATDs is eliminated, and resistance is not fostered.
- For operational ease the R TCP provides separate FDC tablets of I st Iine drugs for use during IP and CP.
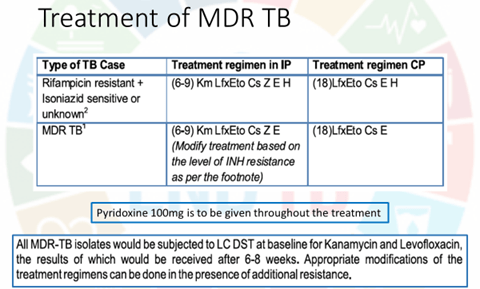
Multidrug-resistant (MDR) TB
- accounts for 3% of all new TB cases and 12- 17% of retreatment cases in different states
- As per WHO global TB report 20 15, India has the highest number of MDR-TB cases
The general principles of treatment of MDR-TB are :
• The regimen should have at least 4 drugs certain to be effective. Often 6 drugs are included, since efficacy of some may be uncertain.
• Reliance about efficacy may be placed on DST results, and the anti-TB drugs used previously in that individual.
• Avoid combining cross resistance drugs, e.g. two FQs, Km with Am or Eto with Pto, or Cs with terizidone.
• Include drugs from group I to group IV in a hierarchical order. Group I drugs (Z, E) can be included , add one injectable drug (group II ), one FQ (group II) and two group IV drugs.
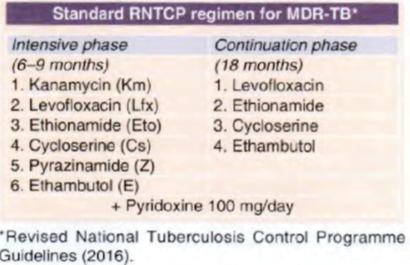
The minimal 6 month intensive phase is extended by I month each time till a maximum of 9 months, if the sputum culture put up at the end of 4 th, 5th and 6th month respectively are positive.
Rifampin resistant TB (RR-TB)
According to both WHO and RNTCP, a case of RR-TB is treated as MDR-TB.
- However, because the bacilli are sensitive to H, this 1st line drug is added to the MDR-regimen, both in the
intensive phase as well as in the continuation phase, without changing the duration or the other
drugs.
- Thus, for RR-TB, the intensive phase of 6 months has 7 drugs (Km, Lfx, Eto, Cs, Z, E and I-1), and the continuation phase ( 18 months) has 5 drugs (Lfx, Eto, Cs, E and H).
Monodrug resistant TB
When the liquid culture drug sensitivity test (LC-DST) or line probe assay ( LPA) reports resistance to one first
line drug, other than R resistance, the treatment regimen according to RNTCP-2016 consists of:
• R + two of the I st line drugs to which bacilli are sensitive + one injectable second line drug + one FQ to make a total of 5 drugs given daily in the intensive phase of 3- 6 months.
Polydrug resistant TB
When the bacilli are resistant to more than one first line drugs, other than R resistance, the initial regimen is :
R + one injectable 2nd line drug + one FQ + any I st line drug to which the bacilli are sensitive – one of the oral 2nd line drugs (Eto/Cs/ PAS). The duration of intensive phase is 3- 6 months.
In the continuation phase for both mono and poly drug resistant TB, the injectable drug is stopped while the remaining 4 oral drugs are continued for a fixed duration o f 6 months .
Thus, the total duration of regimens for mono and poly drug resistant TB is 9- 12 months.
Isoniazid resistant TB
lt has been observed that when H resistance is of low level (in LC-DST) or is due to inhA mutation (in LPA), high dose of I H (900 mg/day for 46-70 kg body weight) in place of 300 mg/ day, is effective and should be added to the regimen (along with pyridoxine).
However, in case of high level H resistance or that due to Kat C mutation, it is ineffective and should not be given.
Extensive drug-resistant TB
- The WHO estimated that 9.7% of MDR-TB patients had XDR-TB in 2015.
- The XDR-TB is very difficult to treat, has a rapid course and high mortality.
- However, to prevent further amplification of resistance, the standard MDR regimen must be immediately
stopped when XDR-TB is detected or suspected.
RNTCP (2016) has recommended a treatment regimen for XDR-TB consisting of 7 drugs in the intensive phase (6-12 months) and 6 drugs in the continuation phase ( 18 months).
The drugs and their adult daily doses (for 46-70 kg body weight) are:
Capreomycin I000 mg
Moxifloxacin 400 mg
High dose isoniazid 900 mg
PAS 12 g
Clofazimine 200 mg
Linezolid 600 mg
Amoxicillin/clavulanate (875+125 mg tab) 2 tab morning + one tab evening.
In the continuation phase, injection capreomycin is stopped and the remaining 6 drugs are continued for another 18 months.
Tuberculosis in pregnant women – RNTCP 2016
standard 6 month (2HRZE + 4HRE) regimen for pregnant women with TB.
S is contraindicated because it is ototoxic to the foetus.
All pregnant women being treated with INH should receive pyridoxine 10- 25 mg/day.
Treatment of breastfeeding women
All I st line ATDs are compatible with breastfeeding; full course should be given to the mother, and breastfeeding should be continued.
Infant à should receive 6 month isoniazid preventive treatment after ruling out active TB followed by BCG vaccination.
mothers are taking INHà supplemented with pyridoxine 5 mg/day.
Latent tubercular infection (Chemoprophylaxis)
indicated only in subjects at high risk of developing active TB, viz.:
(a) Contacts of open cases who show recent Mantoux conversion.
(b) Children with a sputum positive TB patient in the family.
(c) Neonate of tubercular mother.
(d) Patients of leukaemia, diabetes, silicosis or those receiving immunosuppressant medication or those on long-term corticosteroid therapy.
(e) HI V infected contacts of sputum positive index cases.
The standard drug for chemoprophylaxis of TB is INH 300 mg (10 mg/kg in children) daily for 6 months.
Role of corticosteroids
- should not be ordinarily used
- In seriously ill patients (miliary TB or severe pulmonary TB) to buy time for drugs to act.
- When hypersensitivity reactions occur to antitubercular drugs.
- meningeal/renal/pericardial TB or pleural effusion-to reduce exudation, prevent its organisation and strictures
- In AIDS patients with severe manifestations of tuberculosis
- Corticosteroids are contraindicated in intestinal tuberculosis because silent perforation can occur.
Recent advances in TB
Major changes in RNTCP 2016
- Classification of drugs used in TB: (grp 1: First line. Grp 2,3,4: Second line. Grp 5: Third line)
- Grp 1: HRZE, rifapentine, rifabutin
- Grp 2: Inj AG: S, Kanamycin, amikacin. Inj polypeptides: Capreomycin, viomycin
- Grp 3: Oral and Inj FQ: Ciproflox, oflox, moxi, gati, levo
- Grp 4: PAS. Ethionamide, Thioacetazone [PET]
- Grp 5: Clofazamine, Linezolid, Amoxclav, Imipenem and cliastatin, clarithro
- New RNTCP guidelines
- DOTS-Plus
- Added t/t of MDR TB to DOTs.
- 6 (9) Kana, oflox (levo), ethionamide, Cycloserine, ZE/ 18 oflox (levo), ethionamide, Cycloserine, E
- All drugs should be given in a single daily dosage under directly observed treatment (DOT) by a DOT Provider. All patients will receive drugs under direct observation on 6 days of the week. On the 7th day (Sunday) the oral drugs will be administered unsupervised whereas injection kanamycin will be omitted.
- New RNTCP categorization.
- Cat 1. Newly diagnosed case: Sputum positive, sputum negative, extrapul, others
- Cat 2. Previously treated: relapse, failure, defaulter, other.
- T/t. Cat 1: 2 HRZE/4HR. Cat2: 2HRZES 1 HRZE/5 HRE
- Daily therapy rather than intermittent
- HRE in continuation phase than HR
- Follow up – New and previously treated Drug sensitive pulmonary tuberculosis – No need to extend Intensive phase, sputum microscopy at end of IP and end of treatment, weight monthly, chest x-ray if required.
- Introduction of Bedaquiline as a new drug
- Definition and treatment of Mono & Poly-resistance TB apart from MDR & XDR TB
- New algorithm to diagnose Tuberculosis – Pulmonary, Extra-pulmonary, Drug resistant.
- Introduction of Newer molecular methods like Cartridge Based Nucleic Acid Amplificaiton Test(CBNAAT) and line probe assay in diagnostic algorithm apart from smear microscopy and chest Xray.
Daily regimen
☞ Drug sensitive TB : PLHIV, Pediatric TB patients all over county
☞ All TB patient in 104 districts initially
☞ Principle : to administer fixed dose combinations of first line anti-TB drugs in appropriate weight bands
☞ Advantages: Reduced side effects , Improved compliance, Prevent drug resistance, improve drug supply logistics
- New chemical entities
- Bedaquiline
- MOA: ATP synthase has Fo and F1 part. Fo is membrane bound and consists of c ring formed by c proteins. F1 part as α, β, δ, subunits. The γ and ε subunits anchor the F0 portion to F1 portion. Bedaquiline wedges between the ε subunit and c- ring and interfere with the proton translocation step of ATP synthase.
- Added to a 5 drug combination regimen of newly diagnosed MDR TB. Showed faster sputum culture conversion time. Also a higher proportion of patients achieved sputum culture conversion a/c/t placebo.
- Bactericidal. Activity against both DS and DR TB. Equal activity against both replicating and dormant TB. T1/2 of 5.5 months and a very large volume of distribution.
- BBW: increased death. QTc prolongation. S/E: increased hepatic enzymes
- UDFDA gave accelerated approval in Dec 2012 on the basis of Phase 2 b data
- In order to prevent development of resistance to a drug which is the first drug against TB in 40 years WHO has given guidelines for usage of bedaquiline
- when an effective treatment regimen containing four second-line drugs in addition to pyrazinamide according to WHO recommendations cannot be designed
- when there is documented evidence of resistance to any fluoroquinolone in addition to multidrug resistance.
- The following conditions should be met
- Treatment is administered under closely monitored conditions.
- Proper patient inclusion.
- Patient informed consent obtained.
- Adherence to WHO-recommended MDR-TB regimen.
- Pharmacovigilance and proper management of adverse drug reactions and prevention of drug–drug interactions.
- Delamanid
- It is a nitroimidazole. It is activated intracellularly by F420 deazaflavin dependent nitroreductase which converts it to des-nitroimidazole which generates NO free radicals. Aerobic killing is d/t inhibition of mycolic acid synthesis and anaerobic killing is d/t NO poisoning of cytochrome oxidase.
- Faster sputum conversion a/c/t placebo when added to a MDR TB regimen.
- EMEA’s Committee for Medicinal Products for Human Use approved Delamanid on 21st Nov 2013 on the basis of early small clinical trials on the condition that further safety data will be provided.
- Approved for add on to MDR TB regimen when effective t/t regimen cannot be designed d/t resistance or S/E.
New regimen for MDR TB.
- Bangladesh regimen: Addition of Kana and Prothionamide to intensive phase and Gati, E, Z and clofazimine throughout a/c/t standardized regimens. Outcomes were comparable but with lesser S/E. It has been now sponsored by US and UK.
- STREAM regimen.
Pretomanid (PA-824)
- Bicyclic nitroimidazole-like molecule
- Active against both replicating & non-replicating organisms
- Cell wall inhibition (like isoniazid) & respiratory poisoning (like cyanide)
- Inhibits mycolic acid biosynthesis through unknown molecular mechanism
- Respiratory poisoning through NO release
- Safe, Well tolerated, & efficacious at doses of 100-200mg daily
- In phase 3 (NixTB trial)

It is a 8 year strategy document providing for:
- Goals and strategies for the country’s response to the disease during the period 2017 to 2025
- Aims to direct the attention of all stakeholders on the most important interventions or activities that the RNTCP believes will bring about significant changes in the incidence, prevalence and mortality of TB
Four strategic pillars of NSP 2017-2025 are:
DETECT – TREAT- PREVENT- BUILD
Actions to be taken by government for achieving goals of NSP 2017-2025
- Provision of daily dosage regimen for all TB patients in India
- Country wide scale up of new drugs like Bedaquiline
- Effective strategies to ensure Standard of TB Care in India for patients treated in private sector
- To register all patients with e-NIKSHAY
- The MIS system – linkages with the private pharmacy on sale of anti-TB drugs thereby integrating those patients into the MIS.
- To improve the compliance – MoHFW – customized SMS services
- Increase private HCP engagement by giving them incentives for notification of TB patient and successful completion of treatment
- Setting up RNTCP services in public sector undertaking and corporate hospitals
- Expanding ICT (Information and Communication Technologies) support to private sector
