Aromatase Inhibitors
- Androgens are converted to estrogen in the peripheral tissue of post-menopausal females with the help of an enzyme, aromatase.
- The drugs inhibiting this enzyme will decrease the formation of estrogen
- beneficial in the treatment of breast carcinoma.
First-generation
- aminoglutethimide
second generation
- letrozole, anastrozole, vorozole, fadrozole à nonsteroidal or type II à reversible inhibitors of aromatase
- formestane, and exemestane à steroidal or type I à irreversible (suicide) inhibitor
| Generation | Steroidal (type 1) | Nonsteroidal (type 2) |
| First (nonselective) | – | Aminoglutethimide |
| Second (selective) | Formestane | Fadrozole |
| Third (super selective) | Exemestane (Aromasin) | Anastrozole, Letrozole |
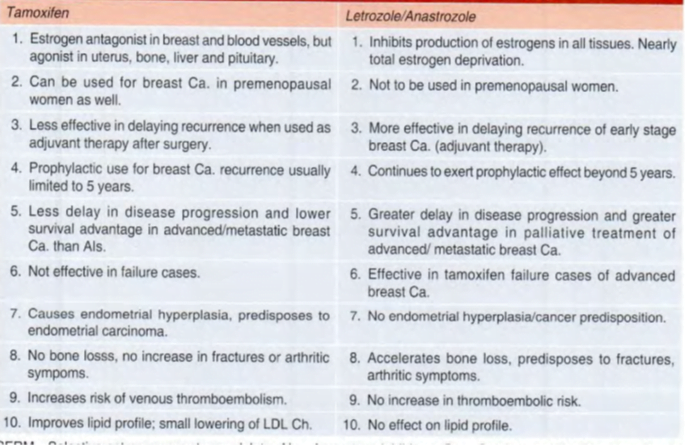
Letrozole
- orally active nonsteroidal (type 2) compound
- reversibly inhibits aromatization including that within the breast cancer cells, resulting in nearly total estrogen deprivation in postmenopausal women
- Als are not recommended in premenopausal women unless ovaries are removed, or Gn secretion from pituitary is suppressed
- The proliferation of estrogen-dependent breast carcinoma cells is suppressed to a greater extent than with tamoxifen.
- rapidly absorbed à 100% oral bioavailability, a large volume of distribution, t½ of ~40 hours.
Uses
Early breast cancer:
- first-line drug for adjuvant therapy after mastectomy in ER+ postmenopausal women.
- Extended adjuvant therapy with letrozole beyond the standard 5-year tamoxifen treatment continues to afford protection.
- Replacement of tamoxifen by an Al is now recommended after 2 years (sequential therapy) because Als do not predispose to thromboembolism and endometrial cancer.
- Survival is prolonged in patients who have positive axillary lymph nodes.
Advanced breast cancer:
- Current guidelines recommend letrozole as first-line therapy because of a longer time to disease progression and higher response rate obtained with it compared to tamoxifen.
- also, effective as a second-line treatment when tamoxifen has failed.
Adverse effects
- Hot flashes, vaginal dryness, nausea, diarrhea, dyspepsia and thinning of hair are the side effects.
- Joint pain and stiffness are common; bone loss may be accelerated.
- However, there is no endometrial hyperplasia or an increased risk of endometrial carcinoma.
- The risk of venous thromboembolism is also not increased, and there is no deterioration of the lipid profile.
- Dose: 2.5 mg OD oral.
- The use of letrozole for inducing ovulation in infertile women has been banned in India since Oct. 20 11.
Anastrozole
- Another nonsteroidal and reversible (Type 2) AI.
- more potent than letrozole and suitable for single daily dosing.
- Accumulates in the body to produce peak effect after 7- 10 days.
- useful as adjuvant therapy in early ER+ breast cancer as well as for palliation of advanced cases in postmenopausal women.
- The risk of a new tumor appearing in the contralateral breast was also lower with anastrozole.
- longer time to disease progression compared to tamoxifen has been obtained in advanced ER+ive breast cancer.
- Many tamoxifen-resistant cases responded with increased survival.
- Like letrozole. it is also a first-line drug for early as well as advanced breast carcinoma in postmenopausal women. Side effects are similar to those with letrozole.
- Dose: 1 mg OD.
Exemestane:
- This steroidal and irreversible (Type I) inhibitor of aromatase acts like a suicide substrate by covalent binding to the enzyme.
- As a result, >90% suppression of estradiol production is obtained.
- However, it has a weak androgenic activity similar to androstenedione.
- found beneficial in early breast cancer by reducing the risk of disease progression when it was substituted for tamoxifen as adjuvant therapy.
- In advanced breast cancer, longer survival, increased time to disease progression and fewer treatment failures have been obtained with exemestane.
- It is administered orally and is well tolerated.
- Adverse effects are similar to other Als.
- Dose: 25 mg OD oral after meals; those receiving CYP3A4 inducer 50 mg OD.