Biotransformation
Biotransformation is the process whereby a substance is changed from one chemical to another (transformed) by a chemical reaction within the body
OR
The series of chemical reactions that occur in a compound, especially a drug, as a result of enzymatic or metabolic activities by a living organism
What constitutes the biotransformation machinery?
Enzymes (Surgeon) can either make alteration in functional groups or can bring about conjugation with certain chemical moieties in order to solubilise the compound.
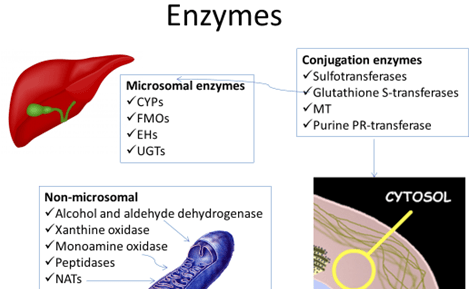
Co-factors (Assistants and nurses) provide reactive species which can be anything from a free electron to an entire functional group.
- e.g. NADPH
- UDP-glucuronate
- S-AM
- PAPS
- Coenzyme A
- Glutathione
- PRPP
Endoplasmic Reticulum (the OT): it provides the work environment for this machinery. Lipid soluble substances spontaneously accumulate here
Phases of biotransformation
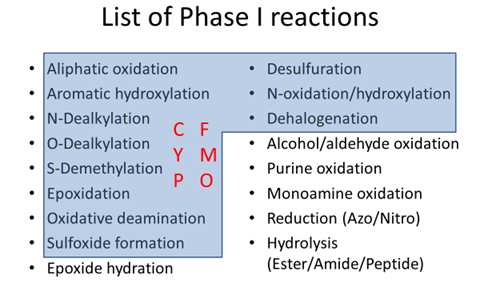
Phase I – (Functionalization) à oxidative and reductive reactions (create new functional groups) and hydrolytic reactions.
They are usually in the direction of increased polarity
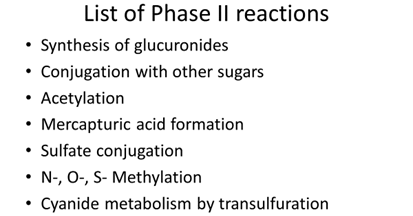
Phase II – drug or metabolite is coupled to an endogenous substrate such as glucuronic acid, acetic acid, sulfuric acid.
Make the drug more polar.
Is it always true that a drug goes through phase 1 followed by phase 2? Ans: No
Gemfibrozil glucuronide is oxidised by CYP
Acetaminophen undergoes direct conjugation to form sulphate and glucuronide.
Some conjugation reactions can even give rise to toxic metabolites like NSAIDS with COOH group form acyl glucuronides which are hepatotoxic.
Hydroxylation
e.g Ibuprofen
Phenytoin administration during pregnancy may produce a constellation of congenital abnormalities,
including cleft palate. This has been ascribed to phenytoin–arene oxide reactivity with cellular DNA in tissues lacking the protective effects of HYL1 which is an mEH.
Dehalogenation and N-oxidation
CCL4 when acted upon by CYP450 yields a trichloromethyl radical which can initiate membrane lipid peroxidation. Alternately, the radical may consume oxygen to ultimately form phosgene.
Halothane, a general anaesthetic agent undergoes both oxidation (90) and reduction (10) forming trifluoroacetic acid and trifluorochloroethane. These reactions are require adequate oxygen tension in the liver which may not always be present during anaesthesia.
Aromatic amines are converted to products that are more toxic than their parent amines are, frequently producing hypersensitivity or carcinogenicity. Dapsone is oxidized by CYP2E1 with high affinity both in vitro and in vivo, and also by CYP3A4. The major side effects of dapsone (methemoglobinemia, agranulocytosis) are linked to its N-oxidation.
Ester and amide hydrolysis
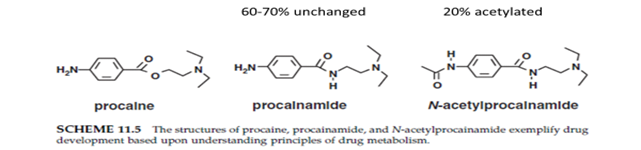
Procaine à antiarrhythmic agent à too rapidly hydrolyzed by esterases to be used in vivo, and its amide analog procainamide à similar to those of procaine and is used clinically as an antiarrhythmic drug. It is relatively resistant to hydrolysis; about 60–70% of the dose is excreted as unchanged drug and 20% is acetylated to N-acetylprocainamide (NAPA), which also has antiarrhythmic activity.
The CYP monooxygenase system
- superfamily of enzymes all of which contain a molecule of heme that is non-covalently bonded to the peptide chain.
- CYPs are associated with the enzyme NADPH-cytochrome reductase (contains both FMN and FAD) in the ratio of 10:1
- Phosphatidylcholine present in the ER membrane is also required for the functioning.
- NH2 terminal of region of the microsomal enzyme anchors CYP protein to the ER.
- This region bears a sequence of hydrophobic aa called signal recognition particle which functions to bind the nascent polypeptide and the associated ribosome to the ER during translation thus ensuring that the newly formed polypeptide is directly inserted into the membrane during the course of its synthesis.
The naming system involves a roman numeral designating a class or a family of forms with an interclass sequence variability of 70% or more.
The proteins within each class are further divided into subclasses denoted by letters when their sequences exhibit a similarity of 70% or more.
This is followed by an identifying arabic numeral for the specific protein.

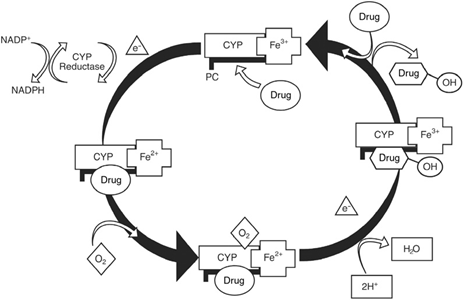
Free drug enters the cycle (upper right) and is complexed to the ferric oxidation state of the heme protein cytochrome P (CYP) in the presence of phosphatidylcholine (PC).
The Fe3+ is reduced to Fe2+ by an electron generated by the conversion of NADPH to NADP+ by the enzyme cytochrome P reductase (upper left).
The reduced complex absorbs molecular oxygen (lower middle). Addition of a second electron from cytochrome P reductase results in the generation of one molecule of water, hydroxylation of one molecule of drug, and the oxidation of iron to Fe3+.
When hydroxylated drug is released from the enzyme complex (upper right), the cycle repeats.
Other microsomal Phase I enzymes
- The Flavin monooxygenases (FMO)
- The Epoxy hydrolases
- FMOs are in the ER. 6 families, FMO3 most abundant. Drugs metab inlcude cimetidine and ranitidine, clozapine, itopride.
- They are minor contributors to metab but are not easily amenable to inhibition or induction and hence are seldom responsible for drug interactions.
- EH à scavenge reactive epoxides capable of damaging DNA, RNA and causing cell toxicity and transformation. They may be competitively inhibited by some substrates. Eg. CBZ interacting with metabolism of valproate (EH inhibitor).
UGTs
- Act on metabolites generated in Phase I reactions rapidly
- The functional groups used for conjugation are alcohol, amino, carbonyl, sulfuryl, carboxyl.
- An important endogenous substrate for these enzymes is bilirubin. Gene polymorphisms can give rise to a number of congenital metabolic disorders like Crigler-Najjar, Gilbert’s syndromes
Enzyme regulation
Enzyme induction and inhibition can affect a drugs half-life, AUC and peak conc. Thus, making pharmacological effects and toxicity unpredictable.
In most cases of enzyme induction, increases in enzyme synthesis result from increased genetic transcription through activation of nuclear receptors. One exception is the induction of CYP2E1 by ethanol, in which ethanol stabilizes the enzyme following transcription, with no effect on receptor-mediated activation.
Enzyme Inhibition
- Reversible
- Competitive
- Non-competitive
- Uncompetitive
- Irreversible
- Quasi-irreversible

Enzyme Induction

Factors affecting
- Gender
Many studies have been conducted in humans and they have all produced equivocal results.
- Age
In humans, activities develop during the first trimester and then plateau until birth followed by a slow rise towards till adult levels are attained.
Ordinarily in adults 90% of Chloramphenicol is glucuronated. Neonates and especially premature infants have very low hepatic glucuronide activity. Plus their glomerular filtration and tubular secretion are also inefficient.
- Liver disease

Significant reductions in CYP2E1 and CYP3A also have been found
- Pregnancy
Pregnancy is an estrogenic state with 100-fold increases in estradiol levels over a woman’s nonpregnant baseline (29, 30). Progesterone, the hormone responsible for sustaining gestation, also dramatically rises during pregnancy from luteal levels of 30–40 ng/mL to levels of 100–200 ng/mL.
BT in New Drug Development
- Analysis by human liver cells/extracts
- Computer based in-silico prediction
- High throughput screening using metabolomics
- Develop biomarkers for routine patient monitoring
