Chirality of Drugs
- Property of a compound whose molecules are not super imposable on their mirror images.
- Chirality = Handedness.
- two isomers are identical in physiochemical properties but different in their ability to rotate plane polarized light.
- Necessary condition for existence of enantiomers.
Significance of Chirality
- Biological activity may reside in only one of the stereoisomer.
- Stereoisomer’s may show essentially similar qualitative and quantitative activity.
- Isomer may exhibit similar qualitatively but different quantitatively activities.
- Isomer may show distinct pharmacological activity.
Types of stereoisomers
- Geometric isomers
- Optical isomers
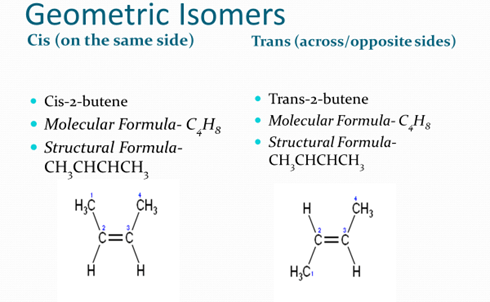
- Because geometric isomers have different chemical and physical properties, they act differently as drugs in our bodies as well.
Example :
- cis-platin used for chemotherapy – it can enter cancer cells and interact with DNA
- Trans- platin – not active
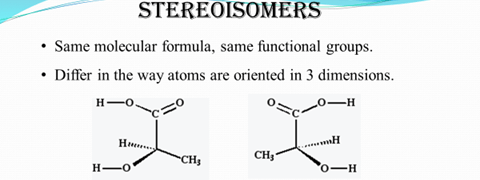
Optical Isomers
- have a chiral center (the central carbon in an optical isomer with four bonds each attached to a different group.)
- Molecule and mirror image cannot be superimposed into each other even after twisting and turning them.
- Identical physical and chemical properties
- Enantiomer: another name for a molecule that is found in optical isomers. For example, ibuprofen has a two enantiomers, a left rotating enantiomer and a right rotating enantiomer.
- Dextrorotatory (“+” / “d” form) – clockwise direction.
- Levorotatory (“-“ / “l” form) – anti-clockwise direction.
- Clockwise – R (rectus).
- Anticlockwise – S (sinister).
- Racemic mixture: A mixture of 50% left rotating and 50% right rotating optical isomers


STEREOPHARMACOLOGY
- Affinity of a drug for a specific receptor and its intrinsic activity are related to its chemical structure.
- Drug – receptor interactions are stereo selective.
- Minor changes in drug molecule structure.
- Major changes in pharmacological properties.
Implication:
- Biomolecules (sugars, amino acids, DNA, proteins, steroids) are chiral
- Proteins are built from L-amino acids, which implies that enzymes – the catalysts of nature – are chiral
- Also, receptors (drug, taste, biopharmaceuticals, agrochemicals) are chiral and the natural ligand to a receptor is often only one specific enantiomer
- This is why mirror image molecules can have radically different activities (effectivity, toxicity, taste) in the body.
- E.g. olfactory sensors are chiral – Different smell
- In 2006 –
- 80% drugs approved by the FDA were chiral.
- 75% single enantiomers.
- FDA now requires information about the structure and activity of each isomer present in a racemic mixture of a new medication.
CHIRAL INVERSION
- Unique metabolic pathway.
- Enzymatic or non-enzymatic.
- Involves unidirectional conversion of one enantiomeric form to another.
R → S
CHIRAL SWITCH
- Development of a single enantiomer from a previously marketed racemate.
- Resulted in a number of agents being re-marketed as chiral drugs.
- Same or similar therapeutic indications.
- Novel indications for old compounds.
THALIDOMIDE
- One asymmetric carbon atom, exists as 2 enantiomers
- S-Enantiomer → sedative
- R-Enantiomer ® Teratogen à Phocomelia
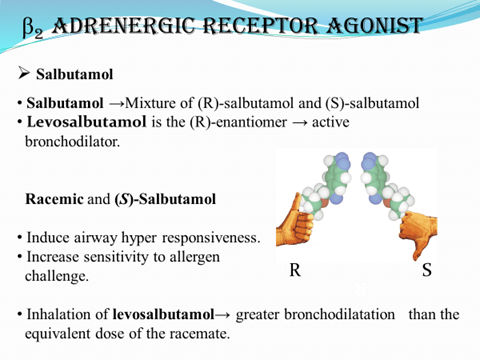
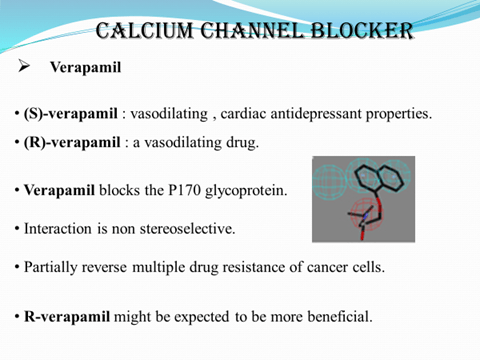
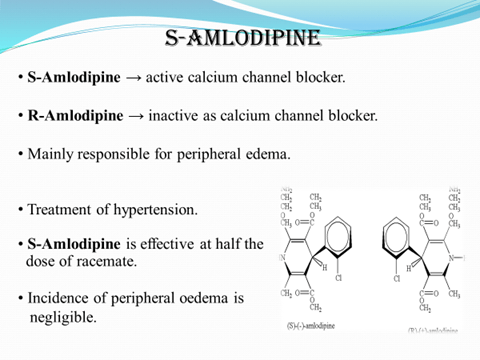
S-Amlodipine is effective at half the dose of racemate
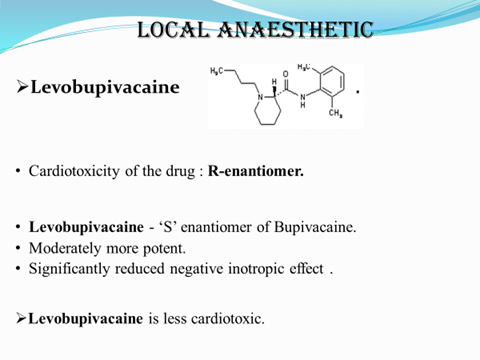
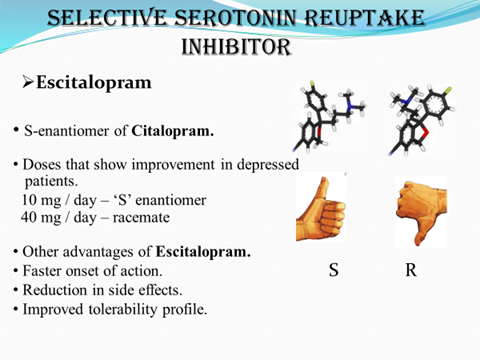
ADVANTAGES OF CHIRAL DRUGS
- Chiral drug is a single agent instead of a mixture of two distinct drugs.
- Simplifies the interpretation of the Basic Pharmacology.
- Greater selectivity for their biological targets improved therapeutic indices and reduced adverse effects.
- Longer or shorter duration of action – more appropriate dosing frequency.
- Decreased inter individual variability.
- SOME DRUGS ARE BETTER AS RACEMATES – b BLOCKERS
