Clinical Trial Design
Clinical trials – Any research study that prospectively assigns human participants or groups of humans to one or more health-related interventions to evaluate the effects on health outcomes
- Interventional trials
Intervention could be à Drugs, cells, and other biological products, surgical procedures, radiologic procedures, devices, behavioral treatments, process-of-care changes, preventive care, etc.
Clinical Trial Design
- Single-arm studies
- Parallel group designs
- Cross over designs
- Enrichment designs
- Add on designs
- Randomized withdrawal designs
- Factorial design
Traditional designs
- involve fixing sample size before the trial commences & performing only single efficacy analysis after the trial has occurred
Flexible designs
- entail actively monitoring efficacy during the trial (either continuously / at intervals) & altering study based on efficacy data
Choice of experimental design has two main objectives:
- Minimize bias, with the aim of obtaining bias-free comparisons
- Minimize the variability of observations, with the aim of obtaining powerful statistical tests & precise estimates
- Single-arm studies
- include an only single group of patients with each patient in the group receiving a single intervention
- No placebo or comparison interventions
- not a parallel-group study
- the anticipated effect of the intervention is large, dramatic, & obvious
- the intervention has not been studied previously
- the study population is homogeneous
- Appropriate patients are very rare
Multiple arms – Parallel group design
- A complete randomized design
- simplest group comparison parallel-group design – the two-group parallel design which compares two treatments
- (e.g., a treatment group vs. a control group).
- Each treatment group – approximately the same number of patients
- use of three-group parallel design: with an active control & placebo control – distinguishes an ineffective drug from an ineffective design
- An effective design: evaluation of assay sensitivity – shows the superiority of active control over placebo
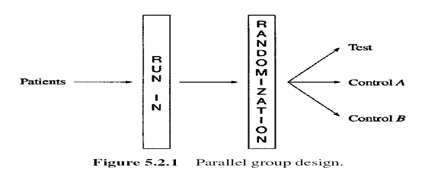
- Run-in (or lead-in) period
- Placebo
- No active treatment
- Dietary control, or active maintenance therapy
Advantage
- washout period to remove effects of previous therapy
- to obtain baseline data and to evaluate if patient fulfills study entry criteria
- training period
- helps in identifying placebo responders
- information regarding patient compliance
When is run in period unsuitable?
- For patients requiring immediate treatment
- Crossover designs
- A crossover design – a modified randomized block design
- Each block receives more than one treatment at different dosing periods
- A block can be a patient or a group of patients
- Patients in each block receive different sequences of treatments
Two periods two sequence cross-over design
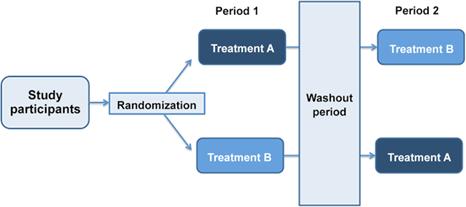
Crossover design – advantages
- It allows a within-patient comparison between treatments since each patient serves as his or her own control
- It removes the inter-patient variability from the comparison between treatments
- With proper randomization of patients to the treatment sequences, it provides the best-unbiased estimates for the differences between treatments
Washout period
- The washout period is defined as the rest period between two treatment periods for which the effect of one treatment administered at one dosing period does not carry over to the next.
- The washout period must be long enough for the treatment effect to wear off so that there is no carryover effect from one treatment period to the next.
Crossover designs may be used if…..
- Chronic (relatively stable) disease are under study
- Drugs with a relatively short half-life are being investigated
- Relatively short treatment periods are considered
- Baseline and washout periods are feasible
- Enrichment
designs
- Therapeutic agents – effective in a specific population of patients
- Identify the patients in whom the test agent is likely to be beneficial in the early phase of the trial
- Enrichment phase – The phase of manipulation of dose levels for identification of patients with drug efficacy
- then randomized to receive either the efficacious dose of the test agent or the matching placebo
- An enrichment design usually consists of at least two phases
The first phase – enrichment phase
- open-label study with a titration design
- to identify patients with a clinical response
The second phase
- Randomized and double-blind, possibly with a concurrent placebo control
- Add-On Design
- In an add-on design, a placebo-controlled trial of a study intervention takes place while patients stay on established effective treatments
- In other words, the trial occurs “on top” of patients receiving regular treatment
- No one ever goes completely untreated
- Placebo patients basically are on established effective treatments
- Unless the study intervention counteracts the effects of the established treatment, patients on the study intervention will be on multiple treatments that could treat the condition
- Allows you to study intervention interactions
Disadvantages of add-on design
- Treatment interactions may occur
- Being on simultaneous treatments can be dangerous
- If a patient improves, Is it due to the study intervention or the established effective treatment?
- The “ placebo ” arm may help but does not always fully answer this question
- Randomized withdrawal design
- A new drug or placebo is added by random assignment to the conventional treatment given at an effective dose, and conventional treatment is then withdrawn gradually, usually by tapering.
- The objective – to compare the ability to maintain the patient’s baseline status between the new drug and placebo.
- Treat all patients with the study intervention in the first period
- Randomize patients to either placebo or continued study intervention treatment for the second period
- Patients remain on the study intervention or placebo until the treatment fails (e.g., symptoms return)
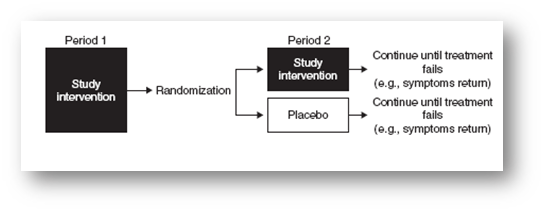
Advantage –
- When long term placebo use is not ethical or practical
- Can evaluate progressive diseases
- Amount of time patient receives less effective treatment is shorter
Disadvantages
- Carryover effects
- Taking patients off treatment abruptly may be dangerous
- Change in the natural course of diseases can confound results
- Factorial designs
- Factorial designs involve testing two or more different interventions on the same group at the same time (i.e., in one period)
- Different groups receive different combinations of the available interventions
- By testing multiple interventions against controls simultaneously, factorial designs minimize the number of patients used
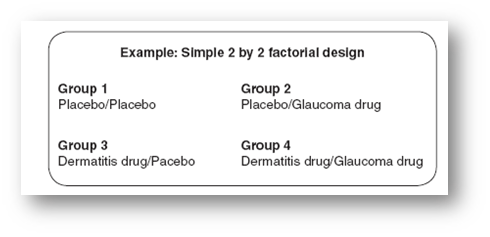
A factorial design can represent:
- Two or more independent studies
- A single study looking at interventions alone and in combination
- Factorial designs can address more than one question in one study in an elegant manner and significantly reduce the required sample size
- The main drawback to the factorial design is the potential for interaction among different interventions
