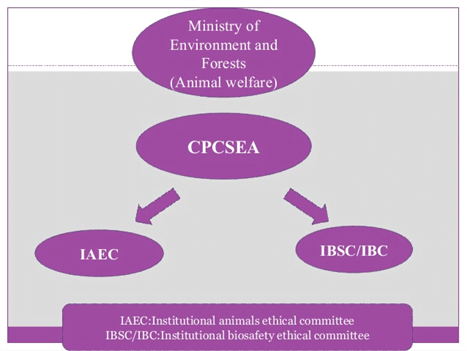
CPCSEA guidelines
- Committee for the purpose of control and supervision of experiments on animals.
- CORE MEMBER: Hon. Smt. Maneka Gandhi – drafted under chairperson
- Headquarters at Chennai
IAEC composition
- A Biological Scientist.
- Two Scientists from different bio disciplines.
- Veterinarians involved in the care of Animals.
- Scientist In-Charge of Animal House Facility.
- The Chairman (preferably Head of the Institution / Department) and
- Member Secretary needs to be nominated from the above five members.
- Other members: Main Nominee, Link Nominee, Scientist from outside and Socially Aware Nominee nominated by CPCSEA.
- The validity of IAEC is for 3 years.
FUNCTION OF CPCSEA
- Registration of establishments conducting animal experimentation or breeding of animals for this purpose.
- Selection and appointment of nominees in the Institutional Animal Ethics Committees of registered establishments.
- Approval of Animal House Facilities on the basis of reports of inspections conducted by CPCSEA.
- Permission for conducting experiments involving the use of animals.
- Recommendation for import of animals for use in experiments.
- Action against establishments in case of violation of any legal norm/stipulation.
Objectives
- to promote the humane care of animals used in biomedical and behavioral research and testing.
- To avoid unnecessary pain before, during and after the experiment.
- To provide guideline for -housing, care, breeding, and maintenance -the source of experimental animals -acceptable experimental procedures for anesthesia and euthanasia
Veterinary care
- Adequate veterinary care must be provided and is the responsibility of a veterinarian or a person who has training or experience ‘in laboratory animal sciences and medicine.
- Observed regularly for the sign of illness, injury, or abnormal behavior
- Contagious disease – isolated from a healthy animal.
- can also contribute to the establishment of appropriate policies and procedures
Animal procurement
- All animals must be acquired lawfully as per the CPCSEA guidelines.
- A health surveillance program for screening incoming animals should be carried out to assess animal quality.
- Methods of transportation should also be taken into account.
- animals should be quarantined and stabilized according to procedures appropriate for the species and circumstances.
QUARANTINE
- Separation of newly received animals from those already in the facility until the health and possibly the microbial status of newly received animals has been determined.
- The minimum duration of quarantine for a small animal-1 week, and
- for a larger animal-6 week.
- An effective quarantine minimizes the chance for the introduction of pathogens into an established colony.
STABILIZATION AND SEPARATION
- Physiologic, psychological and nutritional stabilization should be given before their use.
- The duration of stabilization will depend on the type and duration of animal transportation, and species of animal.
- Physical separation of the animal by species is recommended to prevent interspecies disease transmission and to eliminate anxiety and possible physiological and behavioral changes due to interspecies conflict.
- It shall be acceptable to house different species in the same room, e.g. two species have a similar pathogen status and are behaviorally compatible.
SURVEILLANCE, DIAGNOSIS, TREATMENT, AND CONTROL of DISEASE
- All animals should be observed for signs of illness, injury, or abnormal behavior by animal house staff.
- Animals that show signs of a contagious disease should be isolated from healthy animals in the colony.
- PERSONAL HYGIENE
- Animal care staff maintains a high standard of personal cleanliness.
- It is acceptable to use disposable gear such as gloves, masks, head covers, coats, coveralls, and shoe covers.
- Personnel should not be permitted to eat, drink, smoke or apply cosmetics in animal rooms.
TRANSPORT OF LABORATORY ANIMALS
- main considerations -> • the mode of transport, • the containers, the animal density in cages, food, and water during transit, • protection from transit infection, injuries and stress.
DURATIONS OF EXPERIMENTS
No animal should be used for experimentation for more than 3 years unless adequate justification is provided.
Multiple surgical procedures on a single animal for any testing or experiment are not to be practiced unless specified in a protocol only approved by the IAEC.
LABORATORY ANIMAL: HUSBANDRY & MANAGEMENT
PHYSICAL FACILITIES
Professional judgment must be exercised when developing a practical system for animal care.
Ø Specialized laboratories
Ø Individual areas contiguous with or near animal housing areas for such activities as surgery,
intensive care, necropsy, radiography, preparation of special diets, experimental manipulation, treatment, and diagnostic laboratory procedures containment facilities or
Ø Equipment, if hazardous biological, physical, or chemical agents are to be used
Ø Receiving and storage areas for food, bedding
Ø Pharmaceuticals and biologics and supplies
Ø Space for administration, supervision, and direction of the facility
Ø Showers, sinks, lockers and toilets for personnel
Ø An area for washing and sterilization of equipment and supplies,
Ø An autoclave for equipment
Ø Food and bedding and separate areas
Ø For holding soiled and unclean equipment
Ø An area for repairing cages and equipment
Ø An area to store wastes prior to incineration or removal
- BUILDING MATERIAL à moisture-proof, fire-resistant, seamless materials are most desirable for interior surfaces including vermin and pest resistance.
- CORRIDOR- wide enough to facilitate the movement of personnel as well as equipment and should be kept clean.
- UTILITIES– water lines, drain pipes and electrical connection
- ANIMAL ROOM DOORS– rust, vermin, and dustproof. it properly within their frames and provided with an observation window.
- Windows are not recommended for small animal facilities. However, where power failures are frequent and backup power is not available, they may be necessary to provide an alternate source of light and ventilation
- FLOORS– smooth, moisture-proof, non-absorbent, skid-proof.
- WALLS & CEILINGS- free of cracks, unsealed utility penetrations, or imperfect junction with doors, ceilings, floors, and corners.
- STORAGE AREAS– separate storage areas should be designed for feed, bedding, cages, and materials not in use.
- FACILITIES FOR SANITIZING EQUIPMENT AND SUPPLIES- an area for sanitizing cages and ancillary equipment is essential with adequate water supply.
- EXPERIMENTAL AREA– should be carried out in a separate area from the place where animals are housed.
- ENVIRONMENT • Air conditioning is for laboratory animals.
- a temperature within the range of 18 -29 degrees Celcius relative humidity- 30-70% throughout the year • for large animal comfortable zone-18-37° c, Ventilation designed with 12-15 air cycles per hour.
- POWER & LIGHTING- the electrical system should be safe and provide appropriate lighting and a sufficient no. of power outlets. A time control light system should be used.
- NOISE CONTROL- noise-free environment
ANIMAL HUSBANDRY
- CAGING & HOUSING SYSTEM– Adequate ventilation Meet the biological need of animal Keep the animal dry and clean Cages made of steel or painted steel Feeding and watering devices should be easily accessible for filing, changing, cleaning and servicing.
the number of the animal which can be housed in a particular cage (of different sizes) can be calculated on the basis of (a) floor area of the cage, (b) recommended floor area per animal and (c) weight of animal.
- FOOD • Should be fed palatable, non-contaminated and nutritionally adequate food. • Diet should be free from heavy metals.
- BEDDING Absorbent, free of toxic chemicals or other substances that could injure animals or personnel Should be removed and replaced with fresh materials as often as necessary to keep animals clean and dry.
- WATER • Ordinarily animals should have continuous access to fresh, potable, uncontaminated • drinking water, according to their particular requirements.
- Waste disposal • Wastes should be removed regularly and frequently. All waste should be collected and • disposed of in a -safe and sanitary manner. The most preferred method of waste disposal is incineration.
- EMERGENCY, WEEKEND AND HOLIDAY CARE • Animal should be cared for by qualified personnel every day, including weekends and holidays, to safeguards their well- being including emergency veterinary care
- Recordkeeping The Animal House should maintain the following records:
- Animal House plans, which
include a typical floor plan, all fixtures, etc.
- Animal House staff record – both technical and non – technical
- Health record of staff and animals.
- All SOPs relevant to the animals
- Breeding, stock, purchase, and sales records
- Minutes of institute Animals Ethics Committee Meetings
- Records of experiments conducted with the number of animals used (copy of Form b)
- Death Record
- The clinical record of sick animals.
- Training record of staff involved
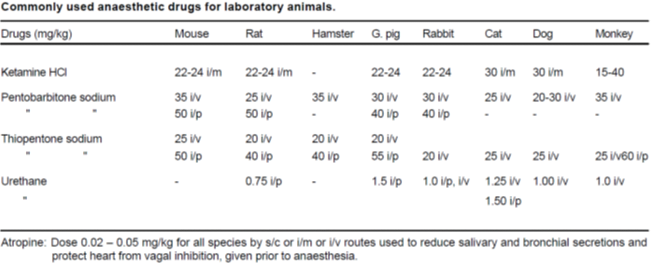
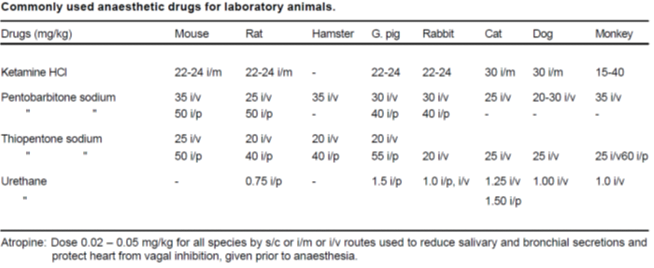
ANAESTHESIA
- Unless contrary to the achievement of the results of the study, sedatives, analgesics, and anesthetics should be used to control pain or distress under the experiment
- ensured that the anesthesia is given for the full duration of the experiment.
- Before using actual anesthetics the animal is prepared for anesthesia by overnight fasting and using pre-anesthetics, which block parasympathetic stimulation of the cardio-pulmonary system and reduce salivary secretion. Atropine is the most commonly used anticholinergic agent
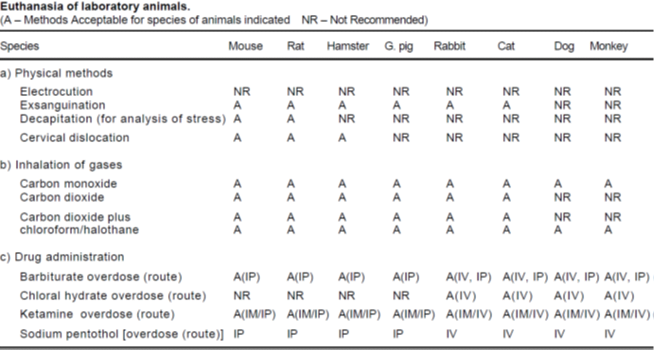
Euthanasia (eu =good, thanatos =death)
- Euthanasia is resorted to events where an animal is required to be sacrificed on termination of an experiment or otherwise for ethical reasons.
- The procedure should be carried out quickly and painlessly in an atmosphere free from fear or anxiety.
- For accepting a euthanasia method as humane it should have an initial depressive action on the central nervous system for immediate insensitivity to pain.
- The method should in all cases meet the following requirements:
- (a) Death, without causing anxiety, pain or distress with minimum time lag phase.
- (b) Minimum physiological and psychological disturbances.
- (c) Compatibility with the purpose of the study and minimum emotional effect on the operator.
- (d) The location should be separate from animal rooms and free from environmental contaminants.
- Methods • Physical methods • electrocution, exsanguination, decapitation, cervical dislocation • Drug administration overdose of chloral hydrate, ketamine, chloroform

Institutional Biosafety Committee (IBSC)
- constituted in all centers engaged in genetic engineering research and production activities.
The committee will constitute the following.
- Head of the institution or his nominee
- 3 or more scientists engaged in DNA work or molecular biology with an outside expert in the relevant discipline.
- A member with the medical qualification-Biosafety officer
- One member nominated by DBT
- Any research project which is likely to have biohazard potential during the execution stage or which
involves the production of either micro-organisms or biologically active molecules that might cause biohazard should be notified to ISBC.
The biosafety functions and activity include the following:
I. Registration of Biosafety Committee membership composition with RCGM and submission of the report.
ISBC will provide half-yearly reports on the ongoing projects to RCGM regarding the observance of the safety guidelines on accidents, risks and on deviations if any.
II. Review and clearance of project proposals falling under the restricted category that meets the requirements under the guidelines.
III. Tailoring biosafety program to the level of risk assessment
IV. Training of personnel on biosafety
V. Instituting health monitoring program for laboratory personnel
Complete medical check-up of personnel working in projects involving work with potentially dangerous microorganisms should be done prior to starting such projects.
Follow up medical check-ups including pathological test should be done periodically, annually
for scientific workers involved in such projects. Their medical records should be accessible to the RCGM. It will provide half-yearly reports on the ongoing projects to RCGM regarding the observance of the safety guidelines on accidents, risks and on deviations if any.
VI. Adopting emergency plans.
So far the biosafety committee has been already set up in 124 institutions. The other institutions will be asked to take similar action.
