Drug use in Pregnancy
Thalidomide à 1957 as an over-the-counter remedy, based on the maker’s safety claims.
Australian obstetrician Dr. William McBride discovered that the drug also alleviated morning sickness. He started recommending this off-label use of the drug to his pregnant patients, setting a worldwide trend. In 1961, McBride began to associate this so-called harmless compound with severe birth defects in the babies he delivered. The drug interfered with the babies’ normal development, causing many of them to be born with phocomelia, resulting in shortened, absent, or flipper-like limbs.
161 babies were adversely affected by thalidomide.
- Special physiological condition
- Total avoidance of pharmacological treatment is not possible
- New medical problems (Gest diab, PIH) can develop and old ones can be exacerbated
- Pregnancy may modify maternal response to drugs, Some effects such as phocomelia seen immed…others such as the adeno ca of vagina was seen after 20 yrs..d/t DES for threatened abortion
Factors affecting drug use in Pregnancy
- Maternal Factors
- Physiological Changes
- Cardiovascular System
Plasma Volume – ↑ses by 40-45%
Heart rate and stroke volume increase in pregnancy leading to a 30–50% increase in maternal cardiac output (CO)
Serum albumin concentration lowered
- Renal System
GFR ↑ses by 25% in 2nd wk to 50 % by 2nd trimester
Renal plasma flow ↑ses by approx. 80%
hypervolemia-induced hemodilution lowers the protein concentration.
- Gastrointestinal System
Reflux of gastric secretions, Due to altered stomach position and decreased lower esophageal sphincter tone.
Nausea/Vomiting
- Pharmacokinetic Changes
- Absorption
- Gastric acid production is decreased during pregnancy, whereas mucus secretion is increased à Increase in gastric pH.
- These changes can increase ionization of weak acids (e.g., aspirin) and reduce their absorption, and weak bases (e.g., caffeine) will diffuse more readily since they will be primarily unionized.
- Drug drug interaction becomes important as antacids and iron may chelate co-administered drugs, which further decreases their already reduced absorption
- Therefore, oral medications should be administered when nausea is minimal.
- Nausea and vomiting in early pregnancy à decrease the absorption following oral administration.
- GI transit time is prolonged.
- Gastric acid production is decreased during pregnancy, whereas mucus secretion is increased à Increase in gastric pH.
- Distribution
- 40- 45% increase in plasma à increased volume of distribution for hydrophilic drugs.
- Body fat expands by approximately 4 kg à increasing the volume of distribution for lipophilic drugs.
- Reduced concentrations of albumin and alpha 1-acid glycoproteinà Decreased protein binding and favors more distribution to tissues.
- Absorption
| ENZYME | PREGNANCY INDUCED CHANGE | POTENTIAL SUBSTRATES IN OBSTETRICS |
| CYP3A4 | Increased | Nifedipine, and indinavir |
| CYP2D6 | Increased | Metoprolol,dextromethorphan, paroxetine,duloxetine, fluoxetine, citalopram |
| CYP2C9 | Increased | NSAIDs,phenytoin,and fluoxetine |
| CYP2C19 | Decreased | Citalopram, diazepam, omeprazole, pantoprazole, propranolol |
| CYP1A2 | Decreased | Theophylline, clozapine, olanzapine, ondansetron |
- Elimination
- GFR is 50% higher by the 2nd trimester and continues to increase until the last week of pregnancy.
- If a drug is solely excreted by glomerular filtration, its renal clearance is expected to parallel changes in GFR.
- For example, the clearance of lithium, which used to treat bipolar disorder, is doubled during the third trimester of pregnancy compared with the non- pregnant state, leading to sub-therapeutic drug concentrations
- Placental factors
- Surface area and thickness of placenta
- Placental blood flow
- Biotransformation reactions in placenta
- Transporters in placenta
placental drug metabolism remains virtually unexplored. Much of the available” data is of a preliminary, exploratory nature and much is also conflicting. It has become evident, however, that in comparison with the liver, the placenta possesses a very limited capacity to biotransform foreign organic molecules.
Three types of drug transfer across the placenta are recognized:
- Complete transfer (type 1 drugs): for example, thiopental
Drugs exhibiting this type of transfer will rapidly cross the placenta with pharmacologically significant concentrations equilibrating in maternal and fetal blood.
- Exceeding transfer (type 2 drugs): for example, ketamine
These drugs cross the placenta to reach greater concentrations in fetal compared with maternal blood.
- Incomplete transfer (type 3 drugs): for example, succinylcholine
These drugs are unable to cross the placenta completely, resulting in higher concentrations in maternal compared with fetal blood.
Properties that Influence Medications Crossing the Placenta
• Molecular weight
• Degree of protein binding
• Degree of ionization (pKa)
• Lipid solubility
• pH of maternal blood influences the degree of ionization
• Fetal to maternal concentration gradient
- Awareness about drug use
- Awareness about the harm drugs can cause to the mother as well as baby
- Education of women to avoid self-medication if they suspect pregnancy
- Nutritional Status
- Nutritional requirements are increased in pregnancy
- Deficiency or excess of nutrients affect expression of genes à may enhance harmful effects of drugs
- Excess of Vit. D is harmful by itself à hypercalcemia & supravalvular aortic stenosis.
- Fetal Factors
- Drugs given to mother at parturition may affect neonate
- Sulphonamides are highly protein bound compete with bilirubin for albumin binding sites, exacerbating neonatal jaundice
- Conjugation capacity of fetal liver is low à Low concentration of UDP glucuronic acid and glucuronyl transferase
- Metabolic & excretory capacity is immature leading to problems with elimination (Near anticipation of delivery)
- Variation in genetic susceptibility to drugs
- Drug Related Factors
- Factors governing drug transfer across placenta
- Dose of drug
- Timing of exposure
- ACE inhibitors Exposure in the 2nd and 3rd trimester of pregnancy is associated with fetal hypotension, renal tubular dysplasia, anuria-oligohydramnios, growth restriction, hypocalvaria, and death
- Past treatment history
- Anti-hypertensives
- Oral hypoglycaemics
- Antiepileptics
- Anticoagulants
- Miscellaneous – Isotretinoin
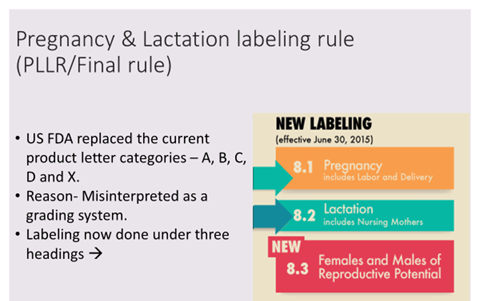
Drug Labeling
- “Pregnancy” and “Lactation” sections will include these subheadings:
- Pregnancy exposure registries
- Risk summary
- Clinical considerations
- Data
- Provide more detailed information regarding human and animal data of the drug and specific adverse reactions.
Pregnancy exposure registries
FDA does not conduct any studies collected in the pregnancy exposure registries and does not endorse any registry; however, the agency may recommend or require that a drug company implement a pregnancy exposure registry based on certain criteria.
TERIS
- Teratogen Information System
- Online database
- Based on thorough review of published clinical and experimental literature.
- Consists of categories such as –
- Unlikely, none or minimal risk – Nifedipine
- Small to moderate – Lamotrigine
- Moderate to high risk – Methotrexate, Phenytoin
- Risk undetermined.