Hematopoietic Growth Factors
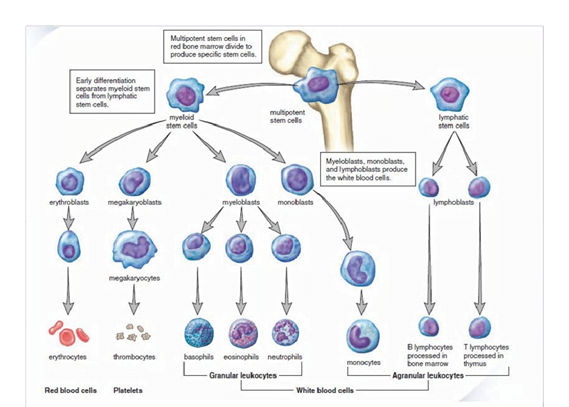
Multipotent stem cells in bone marrow divide to produce specific stem cells , that differentiate into myeloid and lymphatic stem cells…giving rise to respective lineage of erythrocyte, thrombocyte and WBCs.
Hematopoietic growth factors (HGFs) à cytokines that govern haematopoiesis by regulating
- proliferation,
- differentiation,
- maturation,
- function, and
- viability of the cellular components of blood and their progenitor cells.
• production of HGFs is primarily by the cells of the bone marrow except erythropoietin.
- Haematological toxicity is the most common side effect of chemotherapy.
- Several hematopoietic growth factors are currently available for clinical use
- And synthesized mainly by DNA recombinant technology.
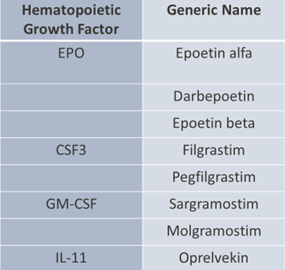
- four major HGFs currently used clinically:
- Erythropoietin (EPO)
- Granulocyte colony-stimulating factor (G-CSF or CSF3)
- Granulocyte-macrophage colony-stimulating factor (GM-CSF)
- Interleukin-11 (IL-11).
- Thrombopoietin is also included, although the currently available clinical agents are thrombopoietin mimetics due to antigenicity of the native cytokine.
- ERYTHROPOIETIN GROWTH FACTORS
- Erythropoietin
- primary regulator of red blood cell production, • primarily produced in the kidney (interstitial fibroblasts and proximal tubular cells)
- • Endogenous EPO levels are depressed in cancer patients – chronic anaemia
- 1977 – purification and cloning -Recombinant human erythropoietin – Epoetin alfa.
- initially approved – anaemia in chronic renal failure patients on dialysis
- later – HIV-related anaemia
- the indication was expanded to include chemotherapy-related anaemia.
- The approval was based on a randomized placebo-controlled trial,
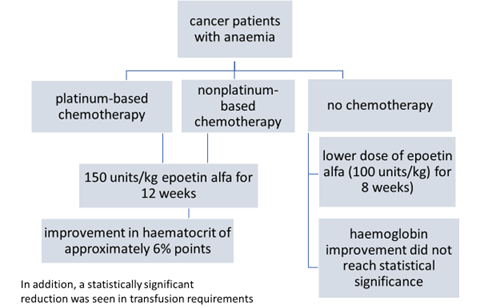
To explore further the impact of anemia and its management on the patient undergoing cancer chemotherapy
- three large open label community-based clinical trials
- anaemia in the setting of chemotherapy
- >7,000 enrolled in the three trials
provided a very robust database
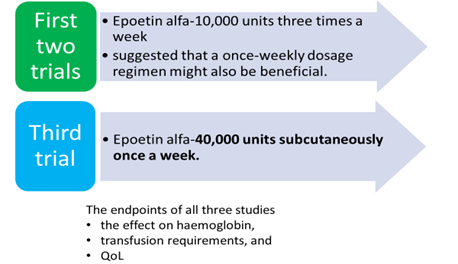
The weekly administration strategy appeared to be as effective as three times a week and has become the clinical standard for administration.
DARBEPOETIN ALFA
- 165 amino acid protein that stimulates erythropoiesis
- differs from erythropoietin and contains 5-N-linked oligosaccharide chains
- increase the molecular weight of darbepoetin alfa to 37,100 Da
- 3 times longer half-life and increased biologic activity.
- 2001- anaemia associated with chronic renal failure
- 2006 – anaemia due to the effects of concomitant myelosuppressive chemotherapy.
Continuous Erythropoietin Receptor Activator (CERA)
- Epoetin beta, third-generation molecule,
- incorporating a large polymer chain
- similar to the previous synthetic EPO drugs, except that it is connected to polyethylene glycol (PEG), which makes it last longer in the body.
- up to 6 times longer than darbepoetin alfa and up to 20 times longer than epoetin.
- 2008- approved for the treatment of aneamia in patients with chronic kidney disease.
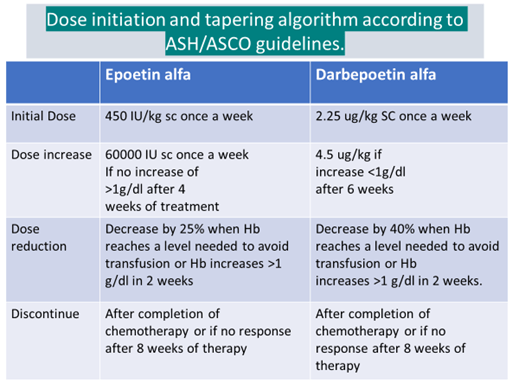
ASCO/ASH guidelines recommendations
- Hb level at initiation- <10g/dl
In patients with Hb 10-12g/dl, ESAs are to be used according to circumstances and clinical judgement
For symptomatic patients with AOC without a reversible cause, transfusion is recommended rather than ESAs
Adverse effects
- Increases the risk of death,
- Myocardial infarction,
- Stroke
- venous thromboembolism and
- tumour progression or recurrence.
- can also lead to an increase in adverse cardiovascular events, hypertension, seizures.
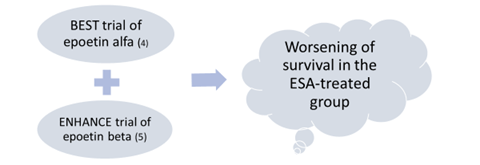
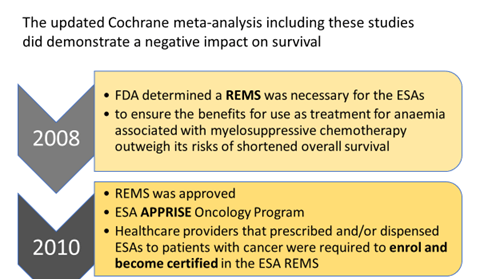
- FDA announced on April 13, 2017 the elimination of the risk evaluation and mitigation strategy (REMS) for ESAs.
By eliminating the REMS:
- Hospitals that dispense ESAs to patients with cancer will no longer be required to enrol and become certified to dispense ESAs.
- While the REMS is no longer necessary to ensure the benefits outweigh the risks, the serious risks of shortened overall survival and/or increased risk of tumour progression or recurrence associated with these drugs remain.
- Health care providers are encouraged to discuss the risks and benefits of using ESAs with each patient before initiating use.
Head-to-Head Study of
Darbepoetin alfa versus Epoetin Alfa à Similar clinical efficacy in
- boosting haemoglobin levels
- reducing the need for red blood cell transfusions and mortality
No differences were observed between the two groups for àhealth-related quality of life assessment
Epoetin alfa is the better pharmacoeconomic value of the two currently available Erythropoietic agents.
Misuse of ESAs
- Erythropoiesis-stimulating agents have a history of misuse by athletes
- The primary reason athletes use ESAs is to improve oxygen delivery to muscles, which directly improves their endurance capacity.
ESAs increase haematocrit and total red cell mass in the body, providing a good advantage in sports where such practice is banned
Myeloid Growth Factors
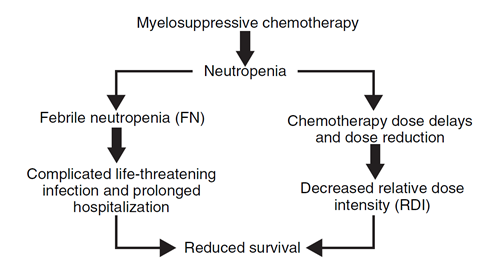
- conventional cytotoxic chemotherapy– most common dose-limiting toxicity- neutropenia and the associated risk of infection.
The likelihood of developing fever in this setting of neutropenia (defined as an absolute neutrophil count [ANC] <500 cells per ml) is approximately 10% per day
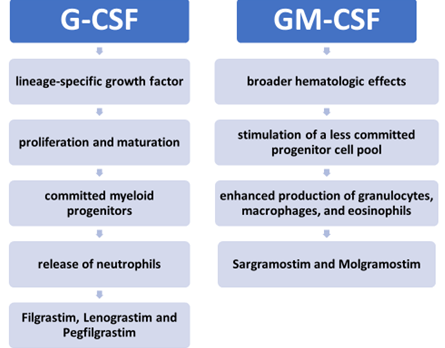
G-CSF
Filgrastim
– produced in Escherichia coli
– has an amino acid sequence identical to endogenous CSF3, with the exception of the addition of an N-terminal methionine and absence of glycosylation.
– The half-life ~ 3.5 hours
Pegfilgrastim
– polyethylene glycol-conjugated version of filgrastim
– The half-life ~ 33 hours
Lenograstim
– a glycosylated recombinant CSF3 derived from a mammalian cell system (Chinese hamster ovaries)
GM-CSF
Sargramostim
- recombinant human granulocyte-macrophage colony stimulating factor (rhu GM-CSF)
- produced in a yeast (S. cerevisiae) expression system.
- The amino acid sequence of Sargramostim differs from the natural human GM-CSF by a substitution of leucine at position 23
- T1/2 is 60 mins
Molgramostim
- an immunostimulant
- prescribed for
- treatment of Ganciclovir-induced neutropenia
- chemotherapy-associated neutropenia
- bone marrow transplantation associated complications.
INDICATIONS AND USAGE
- Cancer Patients Receiving Myelosuppressive Chemotherapy
- Patients With Acute Myeloid Leukemia Receiving Induction or Consolidation Chemotherapy
- Cancer Patients Receiving Bone Marrow Transplant
- Patients Undergoing Peripheral Blood Progenitor Cell Collection and Therapy
- Patients With Severe Chronic Neutropenia
SCHEDULE AND DOSE
- subcutaneous doses of G-CSF- 5 mcg/kg/day
- GM-CSF- 250 mcg/m2/day,
- beginning 24 hours after chemotherapy and continuing until the post-nadir ANC recovery is complete.(10)
- Initial recommendations were to continue the treatment until an ANC of >10,000 cells per ml was achieved, but ASCO guidelines recommend that consideration be given to stopping CSF at ANC levels of <10,000 based on clinical experience and cost considerations.
- The availability of a long-acting formulation of G-CSF has simplified scheduling to a single subcutaneous injection of 6 mg of Pegfilgrastim 24 hours after chemotherapy.
- After administration of these agents à there is a transient decrease in the peripheral WBC count, likely as a result of increased neutrophil adherence to endothelial cells.
- It is estimated that in the presence of G-CSF, this process occurs within 1 day rather than the normal 5-day process in unstimulated bone marrow.
- With the use of GM-CSF, kinetic studies have shown a less dramatic enhancement of proliferation of leukocytes but a prolongation of circulating neutrophil half-life resulting in a sustained leukocytosis.
- In addition to effects on proliferation and maturation of neutrophils, both CSFs enhance
- activities of neutrophils,
- including the respiratory burst,
- adherence,
- superoxide release,
- bacterial killing.
- Adverse effect of CSF
Common
- Gastrointestinal: Nausea and vomiting
- mild-to-moderate bone pain.
- Splenomegaly – reported in chronic use
- transient dyspnoea and pulmonary infiltrates on chest radiography.
- leucocytosis
Serious
- allergic-type reactions
- splenic rupture in persons receiving recombinant CSF3 for peripheral blood stem cell mobilization.
- adult respiratory distress syndrome
- Side effects of G-CSF
- • Better tolerated than GM-CSF
- • Rarely cause Sweet syndrome
- Acute febrile neutrophilic dermatosis
- sudden onset of fever,
- an elevated WBC count, and
- tender, red, well-demarcated papules
Side effects of GM-CSF
• Flu like symptoms
• In vitro evidence that it may stimulate HIV replication but clinical studies not corroborating
GUIDELINE RECOMMENDATIONS
- Primary prophylaxis with a CSF starting with the first cycle and continuing through subsequent cycles of chemotherapy is recommended in patients who have an approximately 20% or higher risk for febrile neutropenia based on patient disease and treatment-related factors.
- Secondary prophylaxis with a CSF is recommended for patients who experienced a neutropenic complication from a prior cycle of chemotherapy (for which primary prophylaxis was not received),
- CSFs should not be routinely used for patients with neutropenia who are afebrile.
- CSFs should not be routinely used as adjunctive treatment with antibiotic therapy for patients with fever and neutropenia.
Precautions of CSF
- • Avoid the simultaneous use of chemotherapy and radiation therapy with G-CSF and GM-CSF;
- DO NOT administer within 24 hours before or after cytotoxic chemotherapy
- – Potential sensitivity of rapidly dividing myeloid cells to cytotoxic chemotherapy.
- • Possible growth stimulation of tumors
HEAD TO HEAD TRIAL
Filgrastim(G-CSF) vs. Sargramostim(GM-CSF)
• no firm evidence indicating the superiority of one over another
- patients who received G‐CSF had faster recovery of ANC
- fewer patients:
- requiring red blood cell transfusions, with fever
- hospital admissions
- less intravenous antibiotic therapy
- There were no significant differences in outcomes between G‐CSF alone and the sequential regimen GM-CSF followed by G-CSF
G‐CSF alone is superior to GM‐ CSF alone for the mobilization of CD34+ cells and reduction of toxicities following myelosuppressive chemotherapy.
Comparative Effectiveness of Filgrastim, Pegfilgrastim, and Sargramostim
- Pegfilgrastim reduces FN incidence to a significantly greater extent than Filgrastim
- Risk of hospitalization for neutropenic complications during cancer chemotherapy is lower with Pegfilgrastim prophylaxis than with Filgrastim or Sargramostim prophylaxis.
- Of the three types of G-CSFs evaluated, Pegfilgrastim seemed to be more cost effective than Filgrastim and Lenograstim.
PLATELETS GROWTH FACTORS
INTERLEUKIN-11
- IL-11 is a bone marrow stroma-derived growth factor
- stimulates haematopoiesis.
- In combination with IL-3, IL-11 promotes megakaryocytopoiesis by stimulating the formation of colony-forming units megakaryocyte and burst-forming units megakaryocyte.
- In addition, IL-11 promotes thrombopoiesis by increasing the size and ploidy of megakaryocytes.
Endogenous IL-11 – Oprelvekin
• Oprelvekin – a rHu IL-11, produced in E. Coli
- half-life was 6.9 ± 1.7 hrs
• Platelet counts begin to rise 5 to 9 days after the initial dose
- Indication-prevention of severe thrombocytopenia and the reduction of the need for platelet transfusions following myelosuppressive chemotherapy in adult patients with nonmyeloid malignancies who are at high risk of severe thrombocytopenia
- not indicated following myeloablative chemotherapy
- 0ther ILs that were demonstrated in model systems to accelerate platelets are IL-3 and IL-6.
- However, early clinical trials of both these molecules were met with significant toxicities.
- IL-11 – more tolerable
- 30% reduction was seen in the need for platelet transfusions among the patients who required platelet support during the previous cycles of chemotherapy when compared with the control group
- the current clinical approval is limited to the secondary prophylaxis setting.
- In clinical practice, IL-11 has been limited in use by side effects that are typical of other ILs with fever, and malaise, as well as other side effects.
THROMBOPOIETIN (TPO)
- humoral regulator of platelet production.
- polyethylene glycol–conjugated truncated form of TPO known as megakaryocyte growth and development factor (PEG-MGDF),
- Stimulates- platelet release.
- In early clinical trials PEG-MGDF and recombinant TPO were successful in shortening the duration of thrombocytopenia, elevating the platelet nadir and reducing the need for transfusion by approximately 30% to 50%.
- Unfortunately, the development of PEG-MGDF was halted after a normal volunteer study revealed the development of cross-reacting antibodies, to native TPO, resulting in prolonged thrombocytopenia.
THROMBOPOIETIN-RECEPTOR AGONISTS
- To avoid the development of auto-antibodies, drug development switched to small molecules in the form of TPO -receptor agonists.
- Romiplostim and Eltrombopag -mimics endogenous TPO
- by binding to and activating the platelet TPO receptor (TPO/R, Mpl, or CD110 antigen).
- Activation of TPO/R stimulates platelet production by proliferation and differentiation of megakaryocytes.
Thrombopoietin Mimetics
- Romiplostim
subcutaneous
• human TPO receptor-binding peptide joined by disulphide bonds to an immunoglobulin Fc fragment
• no homology to endogenous thrombopoietin
• No cross-reactivity (16)
• FDA approved for patients with ITP.
2) Eltrombopag
• oral TPO agonist
• FDA approved for patients with ITP.
• Both are currently being studied in patients with chemotherapy-induced thrombocytopenia
Side effects –
– allergic reactions and anaphylaxis
– Oedema in 2/3 patients,
– Dyspnoea in 50% patients
• At present, platelet transfusion remains the primary therapeutic modality for treatment of thrombocytopenia in the oncology setting.
| Eltrombopag trial data | Romiplostim trial data | |
| Overall response | 67% | 83% |
| Durable response | 47% | 49% |
