Drugs Affecting Coagulation
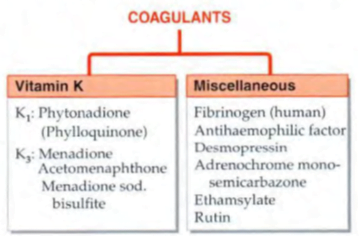
VITAMIN K
- fat-soluble dietary principle required for the synthesis of clotting factors.
- Vit K3 à synthetic
- Dietary sources are—green leafy vegetables, such as cabbage, spinach; and liver, cheese, etc.
- Daily requirement à 50–100 μg/day.
- Action à acts as a cofactor at a late stage in the synthesis by liver of coagulation proteins— prothrombin, factors VII, IX and X.
- Utilization à absorbed from the intestine via lymph
- Vit K is only temporarily concentrated in liver, but there are no significant stores in the body.
- Deficiency à occurs due to liver disease, obstructive jaundice, malabsorption, long-term antimicrobial therapy which alters intestinal flora.
- most important manifestation is bleeding tendency due to lowering of the levels of prothrombin and other clotting factors in blood.
- Haematuria is usually first to occur; other common sites of bleeding are g.i.t., nose and under the skin—ecchymoses.
- only use of vit K is in prophylaxis and treatment of bleeding due to
deficiency of clotting factors in the following
situations:
- Dietary deficiency: very rare
- Prolonged antimicrobial therapy
- Obstructive jaundice or malabsorption syndromes
- Newborns: All newborns have low levels of prothrombin and other clotting factors. Vit K 1 mg i.m. soon after birth has been recommended routinely.
- Overdose of oral anticoagulants: most
important indication of vit K.
- Phytonadione (K1) is the preparation of choice, because it acts most rapidly.
- Prolonged high dose salicylate therapy causes hypoprothrombinemia; vit K should be given prophylactically. If bleeding occurs—treat as for oral anticoagulants.
- Liver disease (cirrhosis, viral hepatitis)
- Menadione and its water-soluble derivatives can cause haemolysis in a dose-dependent manner. Patients with G-6-PD deficiency and neonates are especially susceptible.
- Fibrinogen àThe fibrinogen fraction of human plasma is employed to control bleeding in haemophilia, antihaemophilic globulin (AHG) deficiency and acute afibrinogenemic states
- Desmopressin àreleases factor VIII and von Willebrand’s factor from vascular endothelium and checks bleeding in haemophilia and von Willebrand’s disease
- Adrenochrome monosemicarbazone à It is believed to reduce capillary fragility, control oozing from raw surfaces and prevent microvessel bleeding, e.g. epistaxis, haematuria, secondary haemorrhage from wounds, etc. Its efficacy is uncertain.
- Rutin à plant glycoside claimed to reduce capillary bleeding.
- Ethamsylate à reduces capillary bleeding when platelets are adequate; probably exerts antihyaluronidase action or corrects abnormalities of platelet adhesion, but does not stabilize fibrin (not an antifibrinolytic). Ethamsylate has been used in the prevention and treatment of capillary bleeding in menorrhagia, after abortion, PPH, epistaxis, malena, hematuria and after tooth extraction, but efficacy is unsubstantiated.
LOCAL HAEMOSTATICS (STYPTICS)
- After injury to arterioles and other smaller blood vessels, normal haemostasis occurs successively by contraction of injured vessel wall (lasting few minutes),
- adhesion and aggregation of platelets to form a plug,
- formation of a blood clot, and
- finally in due course dissolution of the clot by fibrinolysis.
- External bleeding is usually stopped by manual pressure, cotton-gauze pressure pack or by suturing.
- Control of bleeding may be aided by local haemostatics (styptics) that are substances used to stop bleeding from a local and approachable site.
- They are particularly effective on oozing surfaces, e.g. tooth socket, abrasions, etc.
- Absorbable materials like fibrin (prepared from human plasma and dryed as sheet or foam), gelatin foam, oxidized cellulose (as strips which can be cut and placed in the wound) provide a meshwork which activates the clotting mechanism and checks bleeding.
- Left in situ these materials are absorbed in 1–4 weeks and generally cause no foreign body reaction.
- Thrombin obtained from bovine plasma may be applied as dry powder or freshly prepared solution to the bleeding surface in haemophiliacs.
- Vasoconstrictors like 0.1% Adr solution may be soaked in sterile cotton gauze and packed in the bleeding tooth socket or nose in case of epistaxis to check bleeding when spontaneous vasoconstriction is inadequate.
- Astringents such as tannic acid or metallic salts are occasionally applied for bleeding gums, bleeding piles, etc.
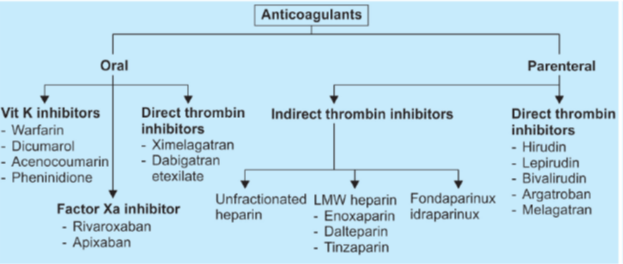
ANTICOAGULANTS
These are drugs used to reduce the coagulability of blood. They may be classified into:
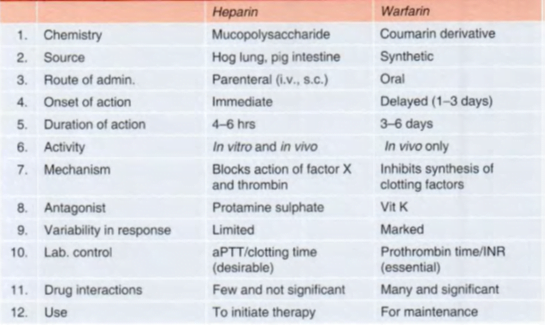
HEPARIN
- Howell and Holt (1918) à ‘heparin’
- non-uniform mixture of straight chain mucopolysaccharides with MW 10,000 to 20,000.
- carries strong electronegative charges and is the strongest organic acid present in the body.
- heparin is present in all tissues containing mast cells; richest sources are lung, liver and intestinal mucosa.
ACTIONS
Anticoagulant
- acts indirectly by activating plasma antithrombin III
- The heparin-AT III complex then binds to clotting factors of the intrinsic and common pathways (Xa, IIa, IXa, XIa, XIIa and XIIIa) and inactivates them but not factor VIIa operative in the extrinsic pathway.
- At low concentrations of heparin, factor Xa mediated conversion of prothrombin to thrombin is selectively affected.
- anticoagulant action is exerted mainly by inhibition of factor Xa as well as thrombin (IIa) mediated conversion of fibrinogen to fibrin.
- Low concentrations of heparin prolong aPTT without significantly prolonging PT. High concentrations prolong both.
- Thus, low concentrations interfere selectively with the intrinsic pathway, while high concentrations affect the common pathway as well.
- Heparin enhances action of AT III by two mechanism
- (a) Long heparin molecule provides a scaffolding for the clotting factors (mainly Xa and IIa) as well as AT III to get bound and interact with each other.
- (b) Heparin induces conformational change in AT III to expose its interactive sites.
- A specific pentasaccharide sequence, which is present in only some of the heparin molecules, binds to AT III with high affinity to induce the conformational change needed for rapid interaction with clotting factors à synthesized and named fondaparinux.
- Inhibition of IIa requires both the mechanisms, but Xa inhibition can occur by mechanism ‘b’ alone. à explains why low molecular weight heparin, which is insufficient to provide a long scaffolding, selectively inhibits factor Xa.
Antiplatelet
- Heparin in higher doses inhibits platelet aggregation and prolongs bleeding time.
Lipaemia clearing
- Injection of heparin clears turbid post-prandial lipaemic plasma by releasing a lipoprotein lipase from the vessel wall à hydrolyses triglycerides of chylomicron and VLDL to FFA.
- This action requires lower concentration of heparin than that needed for anticoagulation.
PHARMACOKINETICS
- Heparin is a large, highly ionized molecule; therefore not absorbed orally.
- Heparin does not cross blood-brain barrier or placenta (it is the anticoagulant of choice during pregnancy).
- Heparin released from mast cells is degraded by tissue macrophages—it is not a physiologically circulating anticoagulant.
- Heparin should not be mixed with penicillin, tetracyclines, hydrocortisone or NA in the same syringe or infusion bottle.
- Heparinized blood is not suitable for blood counts (alters the shape of RBCs and WBCs), fragility testing
Dosage
- conventionally given i.v. in a bolus dose of 5,000–10,000 U, followed by continuous infusion of 750–1000 U/hr.
- The rate of infusion is controlled by aPTT measurement which is kept at 50–80 sec. or 1.5–2.5 times the patient’s pretreatment value.
- Deep s.c. injection of 10,000–20,000 U every 8–12 hrs can be given if i.v. infusion is not possible.
- Needle used should be fine and trauma should be minimum to avoid haematoma formation.
ADVERSE EFFECTS
- Bleeding due to overdose is the most serious complication of heparin therapy.
- Haematuria is generally the first sign.
- Thrombocytopenia is another common problem à generally it is mild and transient; occurs due to aggregation of platelets.
- Transient and reversible alopecia is infrequent.
- Osteoporosis may develop on long-term use of relatively high doses
- Hypersensitivity reactions are rare; manifestations are urticaria, rigor, fever and anaphylaxis.
Contraindications
- Bleeding disorders, history of heparin induced thrombocytopenia.
- Severe hypertension (risk of cerebral haemorrhage), threatened abortion, piles, g.i. ulcers (risk of aggravated bleeding).
- Subacute bacterial endocarditis (risk of embolism), large malignancies (risk of bleeding in the central necrosed area of the tumour), tuberculosis (risk of hemoptysis).
- Ocular and neurosurgery, lumbar puncture.
- Chronic alcoholics, cirrhosis, renal failure.
- Aspirin and other antiplatelet drugs should be used very cautiously during heparin therapy.
Low molecular weight (LMW) heparins
- Heparin has been fractionated into LMW forms (MW 3000–7000) by different techniques.
- LMW heparins have a different anticoagulant profile à selectively inhibit factor Xa
- act only by inducing conformational change in AT III and not by providing a scaffolding for interaction of AT III with thrombin.
- As a result, LMW heparins have smaller effect on aPTT and whole blood clotting time than unfractionated heparin (UFH).
- Also, they have lesser antiplatelet action—less interference with haemostasis.
- Thrombocytopenia is less frequent.
- lower incidence of haemorrhagic complications compared to UFH
- They are eliminated primarily by renal excretion; are not to be used in patients with renal failure.
- more important advantages of LMW heparins are pharmacokinetic:
• Better subcutaneous bioavailability (70–90%) compared to UFH (20–30%): Variability in response is minimized.
• Longer and more consistent monoexponential t½: (4–6 hours); making possible once daily s.c. administration.
• Since aPTT/clotting times are not prolonged, laboratory monitoring is not needed; dose is calculated on body weight basis.
• Risk of osteoporosis after long term use is much less with LMW heparin compared with UFH.
- Most studies have found LMW heparins to be equally efficacious to UFH except during cardiopulmonary bypass surgery, in which high dose UFH is still the preferred anticoagulant
Indications of LMW heparins are:
- Prophylaxis of deep vein thrombosis and pulmonary embolism in high-risk patients undergoing surgery; stroke or other immobilized patients.
- Treatment of established deep vein thrombosis.
- Unstable angina and MI
- To maintain patency of cannulae and shunts in dialysis patients.
LMW heparins à Enoxaparin, Reviparin, Nadroparin, Dalteparin, Parnaparin, Ardeparin,
Fondaparinux:
- produced synthetically
- bioavailability of fondaparinux injected s.c. is 100% and it is longer acting (t½ 17 hours).
- Metabolism is minimal, and it is largely excreted unchanged by the kidney. As such, it is not to be used in renal failure patients.
- less likely to cause thrombocytopenia compared to even LMW heparins.
- Risk of osteoporosis after prolonged use is also minimal.
- Does not require laboratory monitoring of aPTT, and is a longer acting alternative to LMW heparins
DIRECT THROMBIN INHIBITORS
- Unlike heparin, these bind directly to thrombin and inactivate it without the need to combine with and activate AT III.
- Lepirudin àrecombinant preparation of hirudin (a polypeptide anticoagulant secreted by salivary glands of leech)
- Injected i.v., it is indicated only in patients who are at risk of heparin induced thrombocytopenia.
- On repeated/prolonged administration, antibodies against the lepirudin-thrombin complex may develop resulting in prolonged anticoagulant effect and possibility of anaphylaxis.
- Its action cannot be reversed by protamine or any other antidote.
- Bivalirudin à prepared synthetically which has actions and uses similar to lepirudin.
- However, its action is slowly reversible
- Argatroban à produces a rapid and short-lasting antithrombin action. Administered by i.v. infusion, it can be used in place of lepirudin for short-term indications in patients with heparin-induced thrombocytopenia.
HEPARIN ANTAGONIST
Protamine sulfate
- obtained from the sperm of certain fish.
- Given i.v. it neutralises heparin weight for weight, i.e. 1 mg is needed for every 100 U of heparin.
- However, it is needed infrequently because the action of heparin disappears by itself in a few hours, and whole blood transfusion is needed to replenish the loss when bleeding occurs.
- Protamine does not neutralize fondaparinux, and it only partially reverses the anticoagulant effect of LMW heparins.
- Being basic in nature it can release histamine in the body. Hypersensitivity reactions have occurred. Rapid i.v. injection causes flushing and breathing difficulty.
ORAL ANTICOAGULANTS
Action and mechanism
- Warfarin and its congeners act as anticoagulants only in vivo, not in vitro.
- This is so because they act indirectly by interfering with the synthesis of vit K dependent clotting factors in liver.
- behave as competitive antagonists of vit K and lower the plasma levels of functional clotting factors in a dose-dependent manner.
- In fact, they inhibit the enzyme vit K epoxide reductase (VKOR) and interfere with regeneration of the active hydroquinone form of vit K
- This carboxylation is essential for the ability of the clotting factors to bind Ca2+ and to get bound to phospholipid surfaces, necessary for the coagulation sequence to proceed.
- Factor VII has the shortest plasma t½ (6 hr), its level falls first when warfarin is given, followed by factor IX (t½ 24 hr), factor X (t½ 40 hr) and prothrombin (t½ 60 hr).
- Anticoagulant effect develops gradually over the 1–3 days as the levels of the clotting factors already present in plasma decline progressively.
- Thus, there is always a delay between administration of these drugs and the anticoagulant effect.
- Therapeutic effect occurs when synthesis of clotting factors is reduced by 40–50%.
Racemic Warfarin sod.
- most popular oral anticoagulant.
- The commercial preparation of warfarin is a mixture of R (dextrorotatory) and S (levorotatory) enantiomers.
- crosses placenta and is secreted in milk; however, quantity of active form is generally insufficient to affect the suckling infant.
Bishydroxycoumarin (Dicumarol)
Acenocoumarol (Nicoumalone)
Adverse effects
- Bleeding à extension of the desired pharmacological action à causing ecchymosis, epistaxis, hematuria, bleeding in the g.i.t. Intracranial or other internal haemorrhages
- Cutaneous necrosis is a rare complication that can occur with any oral anticoagulant.
- Bleeding is more likely if therapy is not properly monitored, or when INR exceeds 4, or interacting drugs/contraindications are present.
- Treatment: à Withhold the anticoagulant.
- Give fresh blood transfusion à supplies clotting factors
- Alternatively fresh frozen plasma may be used as a source of clotting factors
- vit K1 à specific antidote, but it takes 6–24 hours for the clotting factors to be resynthesized and
- Phenindione produces serious toxicity; should not be used.
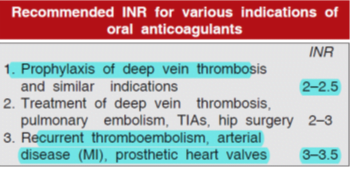
- Dose regulation à A standardized system called the International Normalized Ratio (INR) based on the use of human brain thromboplastin (Tp) has been developed by WHO and adopted in all countries.
Factors enhancing effect of oral anticoagulants are:
• Debility, malnutrition, malabsorption and prolonged antibiotic therapy: the supply of vit K to liver is reduced in these conditions.
• Liver disease, chronic alcoholism: synthesis of clotting factors may be deficient.
• Hyperthyroidism: the clotting factors are degraded faster.
• Newborns: have low levels of vit K and clotting factors
Factors decreasing effect of oral anticoagulants are:
• Pregnancy: plasma level of clotting factors is higher.
• Nephrotic syndrome: drug bound to plasma protein is lost in urine.
• Genetic warfarin resistance: the affinity of warfarin (as well as of vit K epoxide) to bind to the reductase (VKOR) enzyme, which generates the active vit K hydroquinone, is low.
Contraindications à All contraindications to heparin
Warfarin given in early pregnancy increases birth defects, especially skeletal abnormalities. It can produce foetal warfarin syndrome—hypoplasia of nose, eye socket, hand bones, and growth retardation. Given later in pregnancy, it can cause CNS defects, foetal haemorrhage, foetal death and accentuates neonatal hypoprothrombinemia.
Drug interactions
A. Enhanced anticoagulant action
1. Broad-spectrum antibiotics: inhibit gut flora and reduce vit K production.
2. Newer cephalosporins (ceftriaxone, cefoperazone) cause hypoprothrombinaemia by the same mechanism as warfarin —additive action.
3. Aspirin: inhibits platelet aggregation and causes g.i. bleeding—this may be hazardous in anticoagulated patients.
4. Long acting sulfonamides, indomethacin, phenytoin and probenecid: displace warfarin from plasma protein binding.
5. Chloramphenicol, erythromycin, celecoxib, cimetidine, allopurinol, amiodarone and metronidazole: inhibit warfarin metabolism.
6. Tolbutamide and phenytoin: inhibit warfarin metabolism and vice versa.
7. Liquid paraffin (habitual use): reduces vit K absorption.
B. Reduced anticoagulant action
1. Barbiturates (but not benzodiazepines), carbamazepine, rifampin and griseofulvin induce the metabolism of oral anticoagulants.
2. Oral contraceptives: increase blood levels of clotting factors.
DIRECT FACTOR Xa INHIBITORS
- directly bind to and inactivate factor Xa, instead of inhibiting its synthesis.
- They, therefore, act rapidly without a lag time (as in case of warfarin, etc.), and have short-lasting action.
Rivaroxaban
- orally active direct inhibitor of activated factor Xa
- advantage is that it requires no laboratory monitoring of PT or aPTT, and
- recommended in a fixed dose of 10 mg once daily starting 6–10 hours after surgery for prophylaxis of venous thromboembolism following total knee/hip replacement.
- Rivaroxaban has been found equally effective as warfarin for preventing stroke in patients with atrial fibrillation.
ORAL DIRECT THROMBIN INHIBITOR
Dabigatran etexilate
- prodrug à rapidly hydrolysed to dabigatran,
- direct thrombin inhibitor
- no laboratory monitoring is required.
- trial dabigatran etexilate 150 mg twice daily has yielded superior results to warfarin for prevention of embolism and stroke in patients of atrial fibrillation
USES OF ANTICOAGULANTS
- to prevent thrombus extension and embolic complications by reducing the rate of fibrin formation.
- do not dissolve already formed clot, but prevent recurrences.
- Heparin is utilized for rapid and shortlived action, while oral anticoagulants are suitable for maintenance therapy.
- Generally, the two are started together; heparin is discontinued after 4–7 days when warfarin has taken effect.
Deep vein thrombosis (DVT) and pulmonary embolism (PE)
- Because venous thrombi are mainly fibrin thrombi, anticoagulants are expected to be highly effective.
- Prophylaxis is recommended for all high risk patients including bedridden, elderly, postoperative, postpartum, poststroke and leg fracture patients.
- Three months anticoagulant therapy (continued further if risk factor persists) has been recommended by American College of Chest Physicians
- It is based on the premise that inhibition of small amount of activated factor X prevents further amplification of active products—particularly thrombin.
- LMW heparin/fondaparinux have now practically replaced UFH, except in case of major surgery and in high risk cases, because action of UFH can be terminated rapidly.
Myocardial infarction (MI)
- Arterial thrombi are mainly platelet thrombi; anticoagulants are of questionable value. Their use in acute MI has declined.
- They do not alter immediate mortality of MI.
- They may benefit by preventing mural thrombi at the site of infarction and venous thrombi in leg veins. Thus, anticoagulants may be given for a short period till patient becomes ambulatory.
- For secondary prophylaxis against a subsequent attack— anticoagulants are inferior to antiplatelet drugs.
Unstable angina
- Short-term use of heparin has reduced the occurrence of MI in unstable angina patients; aspirin is equally effective.
- Current recommendation is to use aspirin + heparin/LMW heparin followed by warfarin.
Rheumatic heart disease; Atrial fibrillation (AF)
- All atrial fibrillation patients should be protected against thromboembolism from fibrillating atria and the resulting stroke.
- For this purpose, the effective options are warfarin/low dose heparin/low dose aspirin.
- Current guideline is to give warfarin to a target INR of 2–3 in AF patients with high risk for stroke (elderly, heart failure, etc.), and to reserve aspirin for low risk patients or for those unable to take warfarin.
- Anticoagulants are given for 3–4 weeks before and after attempting conversion of AF to sinus rhythm.
Cerebrovascular disease
- Anticoagulants are of little value in cerebral thrombosis.
- They have been used with the aim of preventing clot propagation, but all the trials conducted, including International Stroke Trial (IST), have failed to demonstrate significant benefit.
- Oral anticoagulants may be beneficial in transient ischaemic attacks (TIAs), but antiplatelet drugs are simpler to use and probably better.
- Vascular surgery, prosthetic heart valves, retinal vessel thrombosis, extracorporeal circulation, haemodialysis
- Anticoagulants are indicated along with antiplatelet drugs for prevention of thromboembolism.
Defibrination syndrome or ‘disseminated intravascular coagulation’
- occurs in abruptio placentae and other obstetric conditions, certain malignancies and infections.
- The coagulation factors get consumed for the formation of intravascular microclots and blood is incoagulable.
- Heparin paradoxically checks bleeding in such patients by preserving the clotting factors.
- However, in some cases heparin may aggravate bleeding.
