Peptic ulcers
- A break in mucosal lining of stomach or duodenum >5 mm in diameter, with depth upto submucosa
probably due to an imbalance between
- aggressive (acid, pepsin, bile and H. pylori) and
- defensive (gastric mucus and bicarbonate secretion, prostaglandins, nitric oxide, high mucosal blood flow, innate resistance of the mucosal cell factors)
- Etiology :
- Common: H.pylori infection, NSAID use, Stress
- Uncommon: Zollinger-Ellison syndrome, Hypersecretory states, Tumors, Viral infections
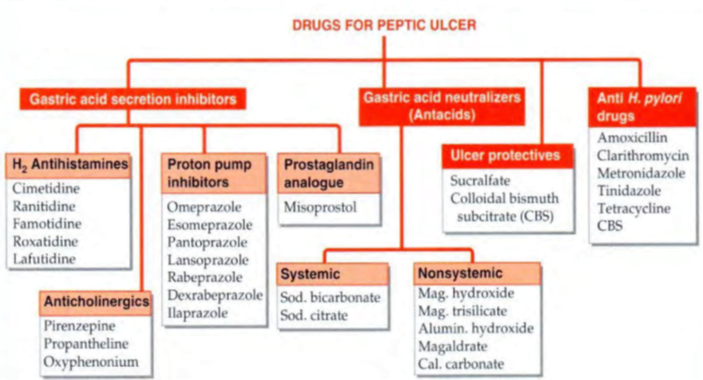
The main strategies employed for the treatment
1. Neutralize gastric acid by antacids.
2. Decrease secretion of acid in stomach.
3. Increase protective factors like mucus and bicarbonate.
4. Protect the ulcer by forming a layer over it.
5. Stimulate the healing of ulcer.
6. Kill H. pylori associated with peptic ulcer disease.
Neutralizing acid secretion à Antacids
- These drugs are weak bases that neutralize gastric acid à do not decrease the volume of acid secreted
- provide prompt relief from ulcer pain.
- may be systemic (absorbed from the GIT) or local (poorly absorbed).
- Sodium bicarbonate is rapidly acting systemic antacid. It is not indicated for long term use because:
–– It releases CO2 that can cause belching and gastric distension (ulcer perforation can occur).
–– Sodium chloride formed in the neutralization reaction can be absorbed that can exacerbate fluid retention in patients of CHF and hypertension.
–– Systemic and urinary alkalosis may occur.
–– Rebound hyperacidity can occur.
• Aluminium hydroxide [Al(OH)3], magnesium trisilicate, megaldrate and magnesium hydroxide [Mg(OH)2] are non systemic antacids.
- These are slower but longer acting drugs. Rebound acidity does not occur.
- Al (OH)3 causes constipation whereas
- magnesium salts are responsible for diarrhea.
- Simethicone à water repellant, pharmacologically inert anti-foaming agent. It reduces flatulence and can also be used to prevent bed sores.
- Antacids decrease the absorption of acidic drugs (acidic drugs are ionized in alkaline medium) and tetracyclines (by forming complexes)
- Milk alkali syndrome (hypercalcemia, renal insufficiency and metabolic alkalosis) may be caused by excessive doses of Na2CO3 or CaCO3 with calcium containing foods (like milk).
Drugs Decreasing Acid Secretion à
Proton pump inhibitors (PPIs)
- These are prodrugs (active moiety is sulfenamide) and
- act by irreversibly inhibiting H+ K+ ATPase in gastric parietal cells.
- drugs include à omeprazole, pantoprazole, esomeprazole, lansoprazole and rabeprazole.
- These drugs are weak bases and can be destroyed by gastric acid.
- To protect them from gastric acid, these are given as enteric coated tablets.
- On reaching parietal cells, active moiety (sulfenamide) is formed and gets trapped. These can inhibit both basal acid output (nocturnal acid secretion) as well as meal stimulated acid output (maximal acid output).
- PPIs are given orally in early morning empty stomach (just before breakfast).
- Pantoprazole, esomeprazole and lansoprazole can be given i.v.
- These drugs have short t1/2 but can inhibit acid secretion for more than 24 hours (hit and run drugs, inhibit proton pump irreversibly).
- drugs of choice for peptic ulcer disease (PUD) due to any Etiology (even NSAID induced).
- also the agents of choice for gastroesophageal reflux disease (GERD) and Zollinger Ellison Syndrome (ZES).
- For prevention of stress induced gastric bleeding, H2 blockers (i.v. infusion) are preferred over PPIs.
- In patients with nasoenteric tube, immediate release omeprazole (by nasogastric tube) is currently preferred.
Adverse effects:
- PPIs are quite safe drugs and have diarrhea, headache and abdominal pain
- Long-term use of PPI is associated with:
–– Subnormal vitamin B12 levels (reduced absorption)
–– Increase in risk of hip fractures (reduced Ca2+ absorption)
–– Increased risk of enteric bacterial infections à C. difficile infections, Bacterial gastroenteritis
–– Pneumonia
• Lanoprazole is most potent PPI and also safest PPI in pregnancy.
• Esomeprazole, lansoprazole and pantoprazole can be given by i.v. route.
- Tenatoprazole à very long acting PPI à can effectively suppress nocturnal acid secretion.
H2 receptor antagonists
- competitively inhibit H2 receptors in parietal cells, thus inhibiting the acid secretion.
- ACh and gastrin act partly by causing the release of histamine, therefore acid secreting capacity of these agents also is decreased by H2 blockers.
- Drugs in this group are cimetidine, ranitidine, famotidine, roxatidine, nizatidine and loxatidine.
- These drugs are more effective for reducing basal (nocturnal) acid secretion (histamine mediated) than stimulated acid secretion (stimulated by gastrin, ACh, as well as histamine).
- These drugs can be used for GERD, PUD, ZES and prevention of stress induced ulcers.
- Cimetidine is not used routinely because:
–– It can cross blood brain barrier and result in mental state changes.
–– It inhibits binding of dihydrotestosterone to androgen receptors that can manifest as impotence in males.
–– It inhibits metabolism of estradiol and increases serum prolactin levels on long term use, thus can cause gynaecomastia (in males) and galactorrhoea (in females).
–– It is a potent inhibitor of CYP enzymes and can increase plasma concentration of warfarin, theophylline and many other drugs.
–– It is the least potent H2 blocker.
• Famotidine is most potent H2 blocker.
• Loxatidine is a non-competitive blocker of H2 receptors.
• Nizatidine also possess anti-AChE activity and can cause bradycardia and enhanced gastric emptyding, negligible first pass metabolism (~100% bioavailability)
Anticholinergics
- Non-selective anti-muscarinic drugs like propantheline and oxyphenonium can be used for decreasing gastric acid secretion.
- However, by increasing gastric emptying time, these drugs prolong the exposure of ulcer bed to gastric acid.
- Further anticholinergic adverse effects like dry mouth, blurred vision, constipation and urinary retention are commonly seen with these drugs.
- Pirenzepine and telenzepine are selective M1 blockers that are preferred antimuscarinic agents for peptic ulcer disease as these are devoid of anticholinergic adverse effects.
Drugs Increasing Protective Factors
- PGE1, PGE2 and PGI2 act as anti-ulcer drugs by increasing the release of mucus and bicarbonate and by increasing the mucosal blood flow.
- PGs also inhibit H+ K+ ATPase and decrease the acid production.
- Misoprostol (PGE1 analogue) is the MOST SPECIFIC drug for treatment and prevention of NSAID induced peptic ulcer (DOC is PPI).
- Enprostil and rioprostil (PGE2 analogue) are other drugs in this group.
- Commonest side effect of PG analogues is diarrhea and colicky abdominal pain.
Ulcer Protective Agents
These drugs form a covering over the ulcer bed that prevents its exposure to gastric acid.
Sucralfate and colloidal bismuth subcitrate
• Sucralfate:
- aluminium salt of sulfated sucrose.
- At pH below 4, its molecules polymerize to form a sticky layer that covers the ulcer base and acts as a physical barrier to prevent acid exposure.
- It can bind phosphates also and can result in hypophosphatemia.
- It should not be given with antacids because it acts only in acidic medium (antacids raise the pH by neutralizing the gastric acid).
- Most common side effect of sucralfate is constipation.
• Colloidal bismuth subcitrate:
- It also forms an acid resistant coating over the ulcer.
- also dislodges H. pylori from the surface of gastric mucosa and kills it.
- Adverse effects include blackening of tongue and bismuth toxicity (osteodystrophy and encephalopathy).
• Rebamipide and Ecabet are cytoprotective drugs acting by increase in PG generation and by scavenging reactive oxygen species.
Ulcer Healing Drugs
Carbenoloxone à obtained from the roots of licorice.
causes epithelisation of ulcer without decreasing acid production
Anti-Helicobacter Pylori Drugs
Helicobacter pylori, a gram-negative rod à gastritis à subsequent development of gastric & duodenal ulcers, gastric adenocarcinoma, gastric B-cell lymphoma
H. pylori infection can be detected by “urea breath test”. It is responsible for relapse of PUD.
H. pylori eradication indications –
As standard care in patients with gastric & duodenal ulcers
Treatment of mucosa-associated lymphoid tissue lymphomas of stomach
Treatment of chronic atrophic gastritis
Presence of intestinal metaplasia /dysplasia (with +ve H. pylori biopsy)
Selection of eradication regimen
- Combination therapy with 2 or 3 antibiotics (plus acid-suppressive therapy)à highest rate of H. pylori eradication
- A PPI or H2-receptor antagonist significantly enhances effectiveness of H. pylori antibiotic regimens containing amoxicillin or clarithromycin
- A regimen of 10 to 14 days of treatment better than shorter treatment regimens
- Patient complianceà Packaging that combines daily doses into one convenient unit is available
- Emergence of resistance to clarithromycin and metronidazole increasingly, an important factor in failure to eradicate H. pylori
Anti- H. Pylori regimens
- Triple therapy × 10–14 days: PPI + clarithromycin 500 mg + amoxicillin 1 g twice a day (metronidazole 500 mg twice a day can be substituted for amoxicillin)
- Quadruple therapy × 10–14 days: PPI + metronidazole 250 mg + bismuth subsalicylate 525 mg + tetracycline 500 mg four times daily
- Sequential therapy: PPI + amoxicillin 1 g twice a day for 5 days followed by PPI + clarithromycin 500 mg and tinidazole/ metronidazole 500 mg twice a day for 5 days
- PPI + amoxicillin 1 g twice a day + levofloxacin 250 or 500 mg twice a day for 10 days
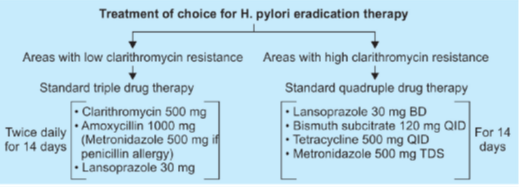
ACG guideline: treatment of H. pylori infection
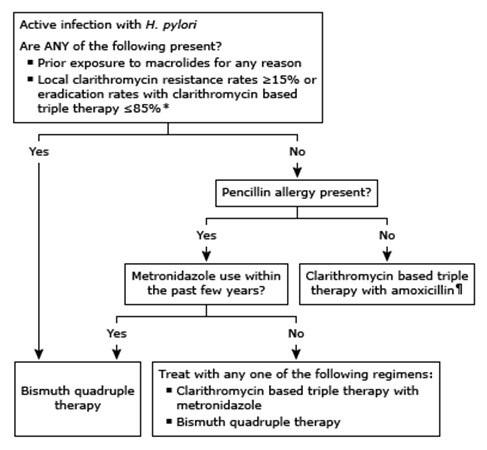
- Bismuth quadruple therapy consists of bismuth, metronidazole, tetracycline, and a PPI.
- Clarithromycin based triple therapy with amoxicillin consists of clarithromycin, amoxicillin, and a PPI.
- Clarithromycin based triple therapy with metronidazole consists of clarithromycin, metronidazole, and a PPI.
CLINICAL LIMITATIONS OF PPIs
- The consistent therapeutic superiority of PPIs over H2RAs, combined with pervasive distribution of these drugs in both over-the-counter and prescription forms, lends itself to the conclusion that these drugs cannot be improved.
- On the contrary, up to 50% of patients taking PPIs for nonerosive GERD are dissatisfied with their treatment due to unresolved symptoms.
- Although a multitude of non-PPI related factors may contribute to this inadequate response (UGI motility disorders, duodeno-gastric-esophageal reflux, visceral hypersensitivity, and patient hyper-vigilance), both the short plasma half-life and necessity for preprandial dosing are significant problems.
- Overnight recovery of gastric acid secretion, termed “nocturnal acid breakthrough,” is frequently encountered with once-daily AM dosing of single release PPIs.
- The escalation of dosing to twice daily is often performed in keeping with both the American Gastroenterology Association and American College of Gastroenterology practice guidelines.
- The importance of timing of drug dosing is also critically under-appreciated. à Approximately half of patients do not take their PPIs within 1 hour of breakfast
- they may not have been instructed to do so by their physician or pharmacist.
- Poor compliance, combined with a narrow window to provide efficacy due to plasma half-life, may be an important cause of PPI failure.
1. Long-term use of PPIs
- Chronic use of high-dose PPIs is thought to
- affect the absorption of calcium, magnesium, and vitamin B12
- Retrospective observational studies and their meta-analyses have also demonstrated a potential link between the use of PPIs and the development of community acquired pneumonia.
- greatest risk was within 7 days of beginning PPI treatment
- A more recent systematic review of trials including only patients prescribed PPI therapy for new-onset NSAID use revealed no increased risk for pneumonia.
- there is evidence that PPI use may enhance patient susceptibility to a multitude of enteric infections including small intestinal bacterial overgrowth, Salmonella, Campylobacter jejuni, and Clostridium difficile Infection with C. difficile (CDI)
- This observation led the U.S. FDA to issue a drug safety communication regarding the importance of PPI exposure, though the impact of both dose and duration of PPI treatment on this association remains unclear.
- All PPIs are, to some extent, metabolized by the cytochrome P450 isozyme 2C19
- Specifically, the potential for PPI induced enzyme inhibition to prevent the activation of clopidogrel has led to a flurry of warnings, revisions, and publications.
- Never the less given our knowledge of the pharmacodynamics, it is our practice to avoid omeprazole (and its stereoisomer, esomeprazole) in patients taking clopidogrel, in favor of alternatives such as lansoprazole, dexlansoprazole, or pantoprazole.
- In 2011 the FDA issued a class warning based on 61 individual case reports indicating that prolonged PPI use could result in low magnesium levels.
- Other recent FDA mandated PPI class warnings include PPI associated acute interstitial nephritis and the possibility of vitamin B12 deficiency with chronic (more than 3 years) daily PPI use.
Advances in PPI technology
- A number of efforts have been made to overcome the inherent pharmacologic limitations of currently available PPIs, in particular their short plasma half-life (and therefore short duration of effect) and the need for preprandial dosing.
Tenatoprazole,
- has demonstrated not only superior inhibitory activity on H+/K+ ATPase, but
- a substantially longer half-life (8 hours after a single dose and 14 after multiple doses) than currently available PPIs.
- This increase in half-life correspondingly increases the area under the plasma concentration curve (AUC) by over 20-fold, representing increased tissue exposure and thus duration of effect at the parietal cell cannaliculus.
Rabeprazole-ER is a 50 mg capsule
- contains 5-distinct 10 mg tablets designed to be degraded and absorbed at intervals throughout the small intestine and colon.
- In a study of healthy subjects, rabeprazole-ER showed superior control of nocturnal gastric acid secretion when compared to both esomeprazole and conventional delayed-release rabeprazole.
Dual-release dexlansoprazole
- formulated to release drug in two separate pH controlled phases.
- Twenty-five percent of the drug dose is liberated in the proximal small intestine at a pH of 5.5, with pharmacokinetics (peak plasma concentration of 1 to 2 hours) similar to traditional enteric coated PPIs.
- This is followed by a second release of 75% of the drug dose in the more distal small intestine at a pH of 6.75 which provides a second serum peak at 5 to 6 hours after administration.
- Like tenatoprazole, this provides an overall increase in the AUC and thereby an overall higher mean 24-hour intragastric pH than its stereo-isomer lansoprazole.
- This offers a potential advantage to patients with obligate twice-daily dosing for symptom control, persistent nocturnal symptoms,or poor compliance with meal timing.
An alternate strategy to avoid the need for premeal dosing would be the inclusion of a stimulator of gastric acid secretion along with the PPI. Succinic acid exhibits pentagastrin like activity and has been FDA approved as a pharmaceutical excipient.
