Drug use in pediatrics
Because children are subject to many of the same diseases as adults, physicians often treat children, by
necessity, with the same drugs and biological products used by adults, even if these products have not been
tested on children.
Dr. Abraham Jacobi, the father of American pediatrics wrote,
“Pediatric does not deal with miniature men and women, with reduced doses and the same class of disease in smaller bodies, but . . . has its own independent range and horizon.”
It is the dynamic process of growth, differentiation, and maturation that sets children apart from adults.
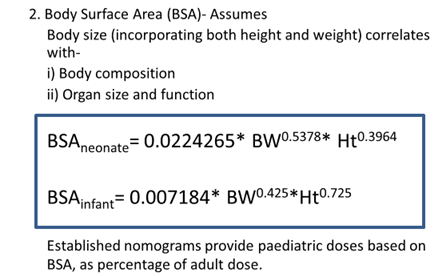
Age related changes in
- Body composition
- Organ functions- determine metabolism and excretion of drugs.
Most dramatic changes in the first 18 months of life…
BA, PK, PD, Efficacy and AE differ markedly-
- Between adult and pediatric population
- Between pediatric patients themselves.
Paediatric patients: 0- 18 yrs of age.
Classifying them-
Preterm: <37 wks
Term: 38- 42 wks
Neonate: <1 month
Infant: 1- 12 months
Toddler: 1- 3 yrs
Children: 3- 12 yrs
Adolescents: 12- 18 yrs
age- dependant changes in the physiologic and biochemical processes govern drug pk and pd.
PHARMACOKINETICS
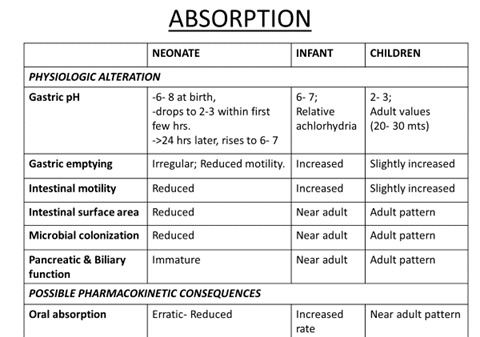
Decreased gastrointestinal motility can delay drug absorption and result in lower peak plasma drug concentrations, but does not alter the fraction of drug absorbed for most drugs.
Gastrointestinal drug absorption
Increased à ampicillin, nafcillin
Decreasedà Phenobarbital, Acetaminophen, Phenytoin
- Drugs given by rectal route:
Preferred in newborns and young infants.
Especially when oral administration impossible.
- Efficient translocation across rectal mucosa.
- Reduced pre- systemic drug clearance.
E.g. Diazepam, Acetaminophen
- Drugs given intramuscularly:
In neonates- premature and term:
- Less muscle mass
- Peripheral vasomotor instability
- Insufficient muscular contractions
- Increased percentage of water per unit of muscle mass.
- Poor perfusion- also in sick infants
Net absorption- Erratic, unpredictable. Not a preferred route.
In infants and children:
Relatively higher density of skeletal muscle capillaries. Perfusion improves.
e.g. Amikacin, Cephalothin.
Drugs given by inhalation:
- Almost instantaneous systemic absorption.
- Avoidance of hepatic fist pass metabolism.
- Local drug delivery.
- Developmental changes in the architecture of the lung and its ventilatory capacity alter the patterns of drug deposition and consequent systemic absorption
e.g. β2 agonists, steroids
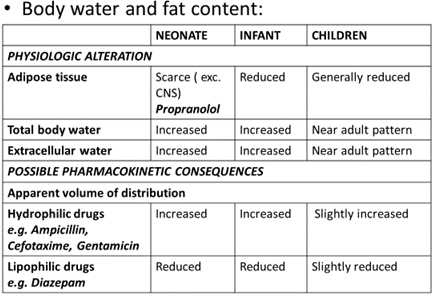
DISTRIBUTION
Age dependant changes in the body composition alter the physiologic spaces into which a drug is distributed.
- Determined by-
- Physicochemical properties of the drug.
- PATIENT FACTORS-
- Extracellular and total body water
- Total body fat
- Plasma protein binding
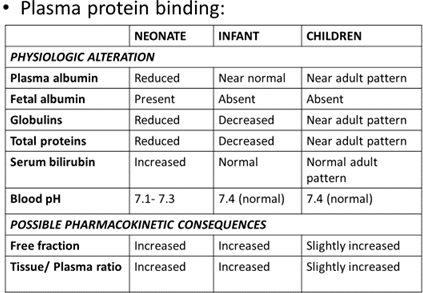
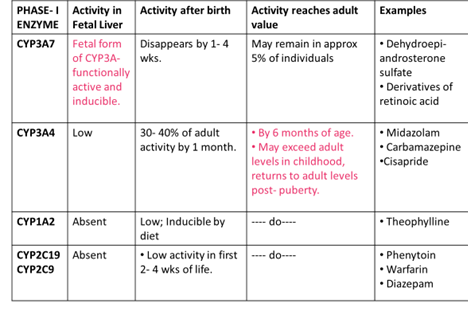
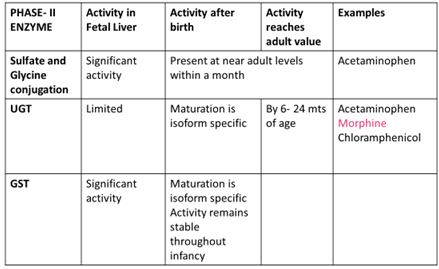
METABOLISM
Most of the enzymatic activities responsible for metabolic degradation of drugs are reduced in the neonate.
The ability to metabolize drugs matures within the first 6- 12 months of life.
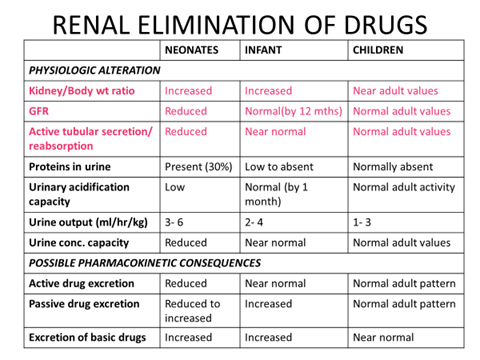
RENAL ELIMINATION OF DRUGS
- Renal function, more than any other organ depends on gestational age and postnatal adaptations…
- Preterm kidneys: Ongoing nephrogenesis.
- Term kidneys: Ongoing functional recruitment.
PHARMACODYNAMICS
- Age-dependent variation in receptor number, receptor affinity for drugs, or the responsiveness of the target organ or tissue to receptor occupancy could influence drug effect.
- Drugs may also alter the growth and development process or express effects that are dependent on the stage of development.
- Opioids à earlier development of opioid receptors specifically in the medulla and pons, where respiratory and cardiovascular centres are located, than in other parts of the brain, is consistent with a clinically observed higher incidence of opioid-related respiratory depression and bradycardia associated with insufficient analgesia in newborns who receive opioids
- Aspirin: Due to the association with Reye’s syndrome, aspirin and other sali-cylates are contraindicated in children and young adults <20 years old with fever associated with viral illness. Reye’s syndrome is characterized by the acute onset of encephalopathy, liver dys-function, and fatty infiltration of the liver and other viscera.
- Midazolam: some children exhibit excitatory effects with midazolam which is an unwanted effect when used as a premedication in the anxious child.
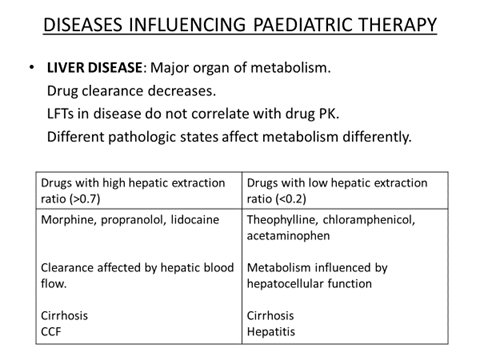
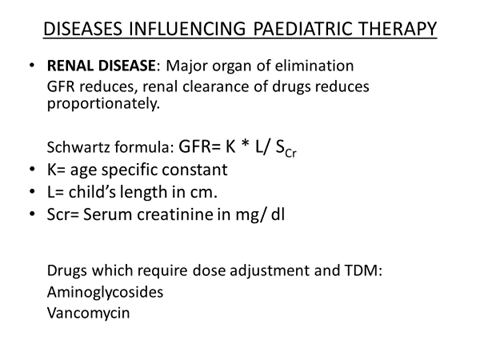
DOSE CALCULATION
Indices used:
- Body Weight: Assumes-
- Total BW correlates with organ size and function.
- Basal metabolism is proportional to BW.

Age (Young’s rule):
Dose= Adult dose * Age (yrs)/ Age + 12
Methods of drug delivery and pediatric formulations:
The form in which a drug is manufactured and the way in which the parent dispenses the drug to the child determine the actual dose administered.
Four children under 36 months died from choking on albendazole tablets during a deworming campaign in Ethiopia in 2007.
Forcing very small children to swallow large tablets may cause choking and asphyxiation.
Recommendations for the administration of such tablets are as follows:
- Scored tablets should be broken into smaller pieces or
- Crushed for administration to young children;
- Older children should be encouraged to chew tablets of albendazole or mebendazole.
- Safe single-dose formulation (e.g. granules or liquid for oral use) to be used.
- Improving the taste of liquid dosage forms- use of flavouring agents, use of ‘chasers’.
Parenteral formulations: Intravenous:
I.V. push- adrenaline, adenosine
Infusion- IVPB (Intravenous piggyback), syringe pumps.
Cautionà Drugs unavailable in pediatric dosage forms. Dilution reqd.- Atropine, morphine, phenobarbital, phenytoin. Measurement errors, stability compromised
Check à Compatibility of two drugs being given through the same site.
- Subcutaneous: Poor blood supply, site of administration- reservoir of drug.
- Intramuscular: Anterolateral aspect of thigh v/s gluteal muscle. >5 yrs- Deltoid muscle
- Ear, Nose and Eye drops:
Warm the medication in the hands for a few minutes before administration; even a product stored at room temperature may feel very cold inside the ears and nose!
Ear drops: Hold the auricle down and out in infants and young children v/s up and back in older children.
Eye and nose drops: Position the child so head is lower than the rest of the body. Restraint while putting eye drops.
OBSTACLES IN PAEDIATRIC RESEARCH
- Ethical hurdles, including the difficulties of obtaining informed consent.
- Need for non-invasiveness.
- Need for microassays, as volumes of samples (e.g. blood) that are available are mostly smaller.
- Stratification of patient population.
- Difficulty in predicting long-term effects during the maturation process.
- Rare diseases (making patient recruitment difficult and small market size providing lower return on investment).
- Necessity for training of paediatricians to assess protocols for research.
- High regulatory requirements.
