Evaluation of anti-arrhythmic
- Cardiac arrhythmias remain a major source of morbidity and mortality in developed countries.
- most common arrhythmia à atrial fibrillation
- Most common cause of sudden death in structural heart disease: VF and sustained VT
- Because the function of many channels is time and voltage dependent, even a drug that targets a single ion channel may, by altering the trajectory of the action potential, alter the function of other channels.
- Most antiarrhythmic drugs affect more than one ion current, and many exert ancillary effects, such as modification of cardiac contractility or autonomic nervous system function.
- Thus, antiarrhythmic drugs usually exert multiple actions and can be beneficial or harmful in individual patients
- Cardiac Action Potential
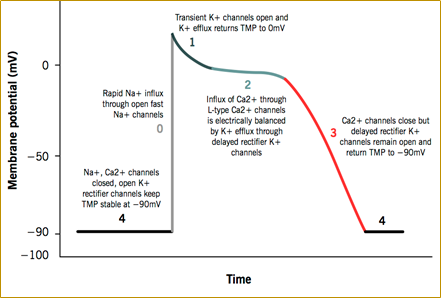
- Mechanisms of Arrhythmia
- Enhanced & abnormal automaticity
- After-depolarizations & triggered automaticity
- Re-entry
- Singh – Vaughan Williams Classification
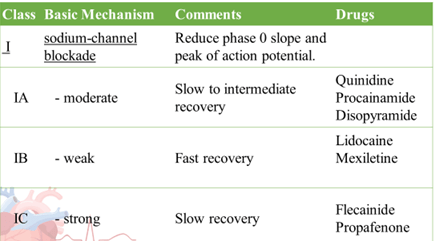
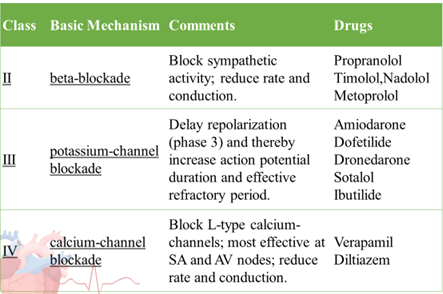
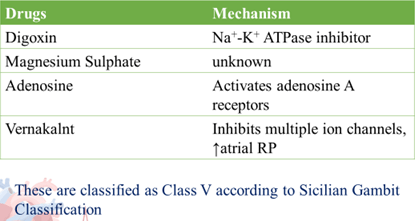
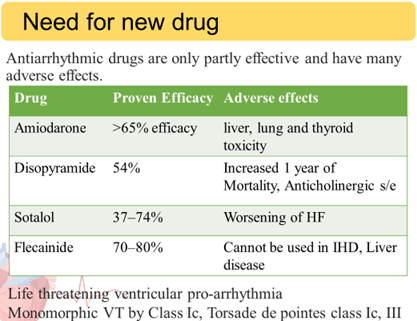
Until now, no AAD has demonstrated reduction in all cause mortality
Ideal Anti-arrhythmic drug
- Effectiveness against a specific group of arrhythmias
- Absence of adverse effects, both cardiac and non-cardiac
- No clinically significant adverse interactions
- Ability to be administered orally as well as intravenously.
- Orally administered drugs should have little or No first-pass metabolism
- No clinically significant polymorphic metabolism between subjects
- Reasonably long half-life to allow less frequent dosing
- Relatively little variability in pharmacokinetic parameters
- Good correlation between its effectiveness and its plasma concentrations.
IN VITRO
- Isolated Guinea Pig papillary muscle
- Studies on Isolated Ventricular Myocytes
- Langendorff Isolated Heart Preparation
- Acetyl choline and potassium induced arrhythmia
- Cell based assays for initial screening
- Isolated GP papillary muscle:
Principle:
- Sodium channel blockade decreases excitability
- Potassium channel blockade lengthens the refractory period
- Calcium channel blockade decreases tension of cardiac muscle
Use: To classify Anti-arrhythmic into Class I, III, IV.
GP sacrificed à Heart isolated.
- The papillary muscle is removed from connecting tissue.
- The upper end is tied to a silk thread and the lower end is attached to a fixed platinum electrode. The other end of the silk thread is tied to a pressure transducer so that isometric contractions are recorded.
- The entire preparation is placed in a organ bath.
- Test drugs are added and then the papillary muscle is stimulated.
- The tension developed is measured: It correlates with the Ca blocking activity of the drug.
- The excitability is measured by recording the strength of stimulus to generate a response: This correlates with the Na channel blocking action
- The Effective refractive period (ERP) is measured by continuous stimulation till a contraction is evoked (The period for which no contraction develops is the ERP): This correlates with the K channel blocking action.
- Studies on Isolated Ventricular Myocytes
- Guinea pigs sacrificed à hearts are removed
- perfused with oxygenated calcium free HEPES bufered saline
- the heart is digested and cut into small pieces, placed in a 20 ml HEPES bufered containing calcium chloride and shaken until single cells are dissociated.
- The cells are then resuspended in HEPES bufered saline and stored
- Transmembrane potential is recorded using conventional glass electrodes connected.
- Cells are stimulated at a frequency of 1 Hz during the stabilization period and at frequencies of 1 and 3 Hz during control and at 10 min after superfusion with test drugs at cumulatively increasing drug concentration
- Concentration response relations are determined by measuring action potentials of currents in each cell during control conditions and during superfusion with two successively increasing concentrations of a given drug.
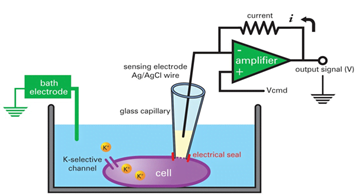
Langendorff technique:
- GP is sacrificed. Heart is isolated and perfused thru a cannula in the aorta. This perfuses the heart retrogradely, which closes the aortic valves like in diastole and the perfusate then enters the coronary arteries.
- The tip of the heart is attached to a hook which is connected to a force transducer which records isometric contractions.
- LAD is ligated and then ligature is released after 10 mins to induce reoxygenation injury.
- Fibrillation can be induced by:
- Simultaneous injection of Digitalis and aconitine
- By lowering the glucose content of the perfusate
- Test drug is added to the medium and the heart is stimulated with a epicardial electrode.
Parameters tested:
- Contractile force, coronary flow and cardiac rhythm.
- The incidence and duration of ventricular tachycardia or
ventricular fibrillation is recorded in the control as well as test group
Applications of Langendorf technique :
- Anti-fibrillating property of drugs
- Calcium antagonism
- Positive and negative inotropic property
Acetylcholine or Potassium induced arrhythmia
- NZ rabbits sacrificed. Atria isolated. Placed in an organ bath.
- The lower end is attached to a electrode and upper end is attached to a thread which is tied to a pressure transducer which records isometric contractions.
- Ach or K is added which induces arrhythmias.
- Test and control drugs are added to the organ bath and time taken to stop the arrhythmias is compared between test and control.
- Test drug is effective if fibrillation disappears immediately or within 5 min following test drug supplementation to the organ bath.
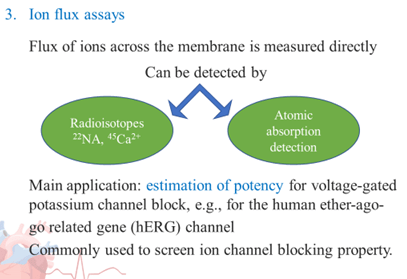
Cell based screening assays
- In such an assay, a radiolabelled (e.g., 3H or 125I)-high-affinity ligand is used.
- This ligand is bound to the channel and can be displaced by unlabelled compound competing for the same site, allowing the affinity (Ki) to this specific binding site to be calculated.
- The ligand-binding technique can be miniaturised to fit into a 384- or even a 1536-well format
In-vivo experiments:
- Chemically Induced arryhthmia:
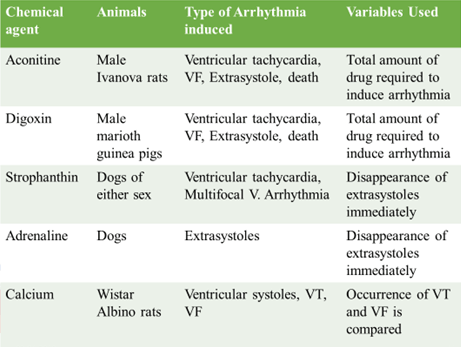
- Aconitine antagonism in Rats
- Digoxin induced arrhythmia in guinea pigs
- Strophanthin/Ouabain induced arrhythmia
- Calcium induced arrhythmia
- Adrenaline induced arrhythmia
- Electrically induced arryhthmia :
- Ventricular Fibrillation Electrical Threshold
- Programmed Electrical Stimulation Induced Arrhythmia
- Mechanically Induced arryhthmia:
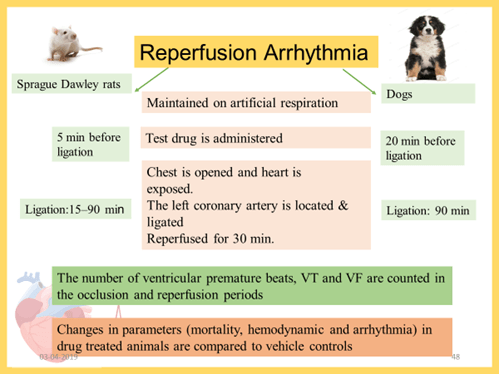
- Reperfusion arrhythmia in rats
- Reperfusion arrhythmia in Dogs
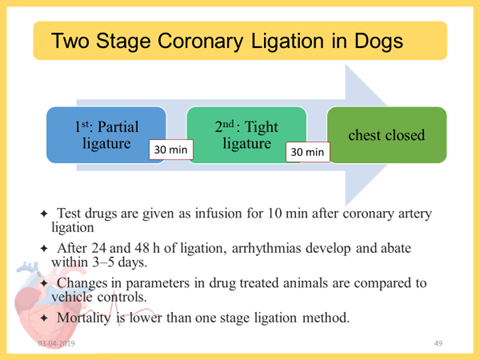
- Two stage coronary ligation in Dogs
- Genetically Induced arrhythmia
- Exercise Induced arryhthmia:
- Ventricular Fibrillation
- Sudden coronary Death model in Dogs
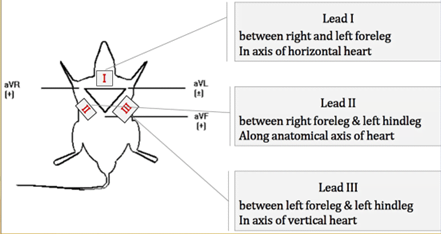
- Recording ECG. Explain lead I, Lead II and lead III.
Chemically induced arrhythmia models
Principle :
- Large number of agents alone or in combination are capable of inducing arrhythmia in experimental animals
- Sensitivity to these arrhythmogenic substances differs among various species.
- Aconitine induced arrhythmias in dogs: Mongrel dogs are taken. Anesthesized. Thorax opened and heart exposed. Femoral vein cannulated to administer drugs. Cotton dipped in aconitine is applied to the atria which induces stable AF or AFl. Test/ control drugs administered till an arbitrary end point is achieved like 1:1 rhythm with HR of 200/min.
Electrically Induced Arrhythmia
- Principle:
- Serial electrical stimulation results in flutter and fibrillation and it is possible to reproduce some of the main types of arrhythmias of clinical importance.
- The flutter threshold or the ventricular multiple response thresholds, may be determined in anesthetized dogs before or after administration of test drug.
- electrical stimulation induced AFl in dogs:
- Mongrel dogs are taken and anesthesized. Thorax opened.
- Pericardium is opened and stitiched to the periphery so that the heart is in a pericardial swing.
- Turn the dog to the left to expose the atrial which is between SVC and IVC. A hemostat is applied on the atria between SVC and IVC to induce tissue damage.
- This damaged tissue is stimulated electrically to induce AFl.
- Test/control drugs are administered via the femoral vein and time taken for reversal to normal sinus rhythm is recorded and compared.
- Various stimuli to measure thresholds:
- Single pulse stimulation
- Train of pulses stimulation
- Continuous 50-hz stimulation
- Sequential pulse stimulation
Mechanically Induced Arrhythmia
- Arrhythmias can be induced directly by ischemia or by reperfusion.
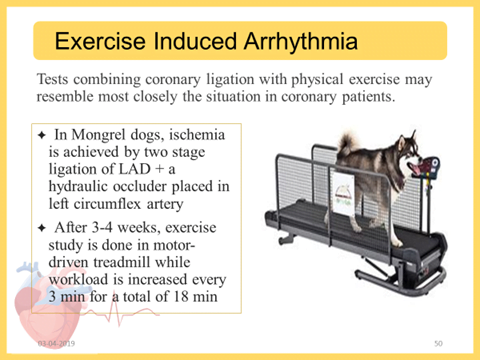
- During the last minute of exercise, the left circumflex coronary artery is occluded, the treadmill is stopped and the occlusion is maintained for 1 additional min
- The occlusion is immediately released if VF occur.
- Test drug given before exercise testing
The effect of test drug is compared to standard drug and control groups using appropriate statistical tests
Advantage: Resembles most closely the coronary heart disease
Genetic arrhythmia
- A colony of German shepherd dogs has been described with inherited ventricular arrhythmias and a predisposition for sudden death
- Most often occurs during sleep or at rest after exercise or excitement.
- These dogs can be used to screen potential anti arrhythmic drugs.
- Transgenic mice deficient for the potassium channel interacting protein KChIP2, a component of the transient outward potassium current (Ito)
- Mice with genetic alterations in the Scn5a gene encoding the cardiac sodium channel-Long QT syndrome type 3, Brugada syndrome, and cardiac conduction disease, inherited conditions associated with sodium channel mutations
- Zebra fish model
- Clinical evaluation
- Methods to assess Efficacy
- ECG- (Rest and exercise) 12 lead ECG measuring automaticity and conduction, ventricular recovery period
- Holter monitoring with/without HM diary
- Exercise testing: When there is suggestion that drug’s action is abolished or intensified by exercise
- Trans telephonic monitoring
- Patients equipped with ICD’s– no of times the ICD has delivered shocks can be calculated
- (ICD) is a battery-powered device placed under your skin, beneath the collarbone, that monitors your heart rate
- Reduction in the no of Fibrillation episodes and sustained VT can be calculated.
Phase I study
- Objective: Safety and Tolerability (Efficacy)
- Outcome measures:
- Primary
Change from of the following:
- Physical examination
- Haematology parameters
- Urine analysis
- 12 lead ECG
- Vitals: BP, HR, Temp
- Telemetry ECG Time: To commence approximately 10 min before dosing up to 6 h post-start of infusion
- Holter ECG with 12 extractions
- Adverse Events
Secondary measures
Cmax, Tmax, AUC0-t, AUC0-inf,
- Subjects: Healthy volunteers (Patients)
- Important exclusion criteria:
- Clinically significant abnormal biochemistry, haematology, coagulation or urine analysis as judged by the investigator, including:
- Serum K <3.5 mmol/L
- Serum magnesium concentration of <0.7 mmol/L
- Serum phosphate <2.5 or >4.5 mg/dL
- QTcF interval >450 or QRS >120 msec
Phase II & III
Efficacy Outcomes:
- Rates of progression of paroxysmal atrial fibrillation to persistent or permanent atrial fibrillation
- Time to first recurrence of atrial fibrillation/flutter
- Atrial fibrillation burden: Counting the number and duration of AF events is an attractive measure of overall AF burden
- Reduced Hospitalizations : both efficacy and safety
- Secondary: Prevention of progression of AF to a more sustained variety, Time to first symptomatic and ECG confirmed recurrence of AF or an adverse event
Endpoints in studies of drugs for pharmacological cardioversion
- Proportion of patients converted to sinus rhythm
- Subsequent time to recurrence
- Recurrence rates
Endpoints in studies of drugs for ventricular rate control
- Mean ventricular rate during rest and at a level of activity that can be achieved by the patients
- Mean heart rate during 24 h Holter monitoring can be used, with inclusion of the minimum and maximum ventricular rates and other Holter-derived parameters as secondary endpoints.
Quality-of-life measures
- Atrial Fibrillation Effect on Quality-of-Life (AFEQT)
- AF-QoL
Cognitive function
Health economics
