Evaluation of drugs in the management of bronchial asthma
Problem statement
- Around 300 million people around the world have asthma.
- Number of disability-adjusted life years (DALYs) lost due to asthma worldwide has been estimated to be currently about 15 million per year – which is 1% of worldwide DALYs lost.
- In India 3% population is suffering from bronchial asthma.
Adverse effects –
LABAs – increased mortality in salmeterol groups compared to placebo, with more in African Americans. Increased the risk of severe exacerbations driven by the number of asthma related hospitalizations, especially in children aged 4 to 11 years.
Budesonide/formoterol combination – SMART trials included respiratory tract infection, pharyngitis, rhinitis, bronchitis, sinusitis, headache, and aggravated asthma. (Sequential, multiple assignment, randomized trial (SMART) designs)
Omalizumab – Anaphylaxis, atherothrombotic events.
2009, the package inserts for montelukast, zafirlukast, and zileuton were updated to include neuropsychiatric events – association with suicide and suicidal events.(0.01%)
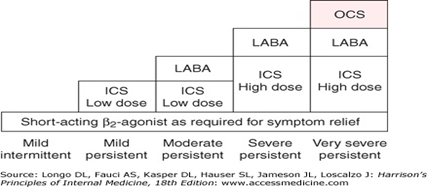
Need for new drugs and strategies
- more than half patients have poorly controlled asthma because of poor adherence.
- Poor compliance is due to lack of immediate symptom relief as seen with anti-inflammatory drugs like CS.
- Burden on healthcare
- Target single mediator, asthma is multifactorial
In Vitro assay
- Histamine (H1) receptor binding
- Spasmolytic activity in trachea
- Spasmolytic activity in isolated guinea pig lung strips
- Reactivity of the isolated perfused trachea
- Bronchial perfusion of the isolated lung
- Inhibition of histamine release from mast cells
- CULTEX
Histamine Receptor binding assay:
- GP sacrificed.
- Brain isolated, homogenized and centrifuged in Tris buffer. The pellet is resuspended in Tris buffer.
- In a shaking bath 3H pyrilamine, test compound and brain membrane suspension and incubated in Tris HCl buffer.
- Then it is filtered and the brain membrane radioactivity is measured by liquid scintillation counter.
- If the test drug has affinity for H1 receptor then it displaces the 3H pyralamine.
Spasmolytic activity on GP isolated tracheal chain:
- The isolated tracheal chain of guinea pigs can be used to test for β -blocking activity
- Used to detect β -sympathomimetic, H1 -receptor blocking and leukotriene receptor blocking properties of test drugs
- GP sacrificed. Trachea isolated and individual tracheal rings cut.
- 10-15 rings are stitched together using silk thread and suspended in an organ bath.
- Histamine or carbachol is added to induce contractions which are allowed to reach a plateau.
- This is followed by adding a test drug and relaxation induced by these drugs are compared to control.
Spasmolytic activity of the GP lung strips:
- Drugs are tested for their capability of inhibiting bronchospasm induced by histamine or calcium ionophore.
- To detect H1 – and leukotriene receptor blocking properties of test compounds
- GP sacrificed. 5 cm lung strips are taken and suspended in an organ bath.
- Contraction is induced by carbachol, histamine or leukotrines.
- This is f/b adding test drugs which if having spasmolytic activity induce relaxation.
- Test modification – Inhibition of prostaglandin synthesis
- Prostaglandin synthesis is inhibited by addition of indomethacin at 10–6 g/ml prior to spasmogen administration.
Reactivity of the isolated perfused trachea
- Mechanism by which the epithelium affects the reactivity of tracheal musculature can be studied
- Contractile agonists can be added either to the serosal (extraluminal) or to the mucosal (intraluminal) surface
- Organ chamber – modified Krebs-Henseleit solution
- Serosal compartment → Trachea placed
- Mucosal compartment → solution pumped at a constant rate of 30 ml/min through the lumen
- Responses of the tracheal musculature are obtained by measuring changes in inlet-outlet ΔP
- Agonists are added in step-wise increasing, cumulative concentrations.
Bronchial perfusion of isolated lung:
- GP is sacrificed and the lung along-with the trachea is isolated.
- One lung is closed off by ligating its primary bronchus.
- The other lung’s lower lobe is cut off and the entire surface of the remaining lung is scratched for facilitating flow of perfusate.
- A three-way cannula is used, one end inserted into the trachea, one end is used for perfusing liquid and the third end is connected to the pressure transducer which measures the pressure.
- Perfusate is infused at a constant rate and the pressure in the pressure transducer is maintained at 500-650 mm of column height.
- Histamine is added to the perfusate which increases the pressure indicated by the increase in the height of the column.
- This is followed by adding the test drug and change in pressure is recorded and compared to control.
- The method has been used to evaluate sympathomimetic drugs.
Inhibition of histamine from mast cells:
- Mediators responsible for hypersensitivity reactions are released from mast cells.
- An important preformed mediator of allergic reactions found in these cells is histamine.
- Specific allergens or the calcium ionophore induce release of histamine from mast cells.
- The histamine concentration can be determined with (fluorescence detector)
- Wistar rats are decapitated and exsanguinated.
- Hanks balanced salt solution (HBSS) is injected into the peritoneal cavity and massaged.
- Then the peritoneal cavity is opened and the peritoneal fluid is centrifuged and the final mast cell concentration is achieved at 105 cells/microL. The histamine conc is determined by fluorescence detector.
- Histamine release by histamine releasers: Histamine release by mast cells after adding histamine releasers like calcium ionophore
- Sample histamine release: test drug is added to mast cell suspension and incubated at 37 C for 15 minutes f/b adding histamine releaser like the calcium ionophore, or a specific allergen.
- Spontaneous histamine release: Histamine release by Mast cells without adding any of chemical.
- % inhibition of histamine release: [(sample H release- spontaneous H release)X100]/(H release by releasers-spontaneous H release)
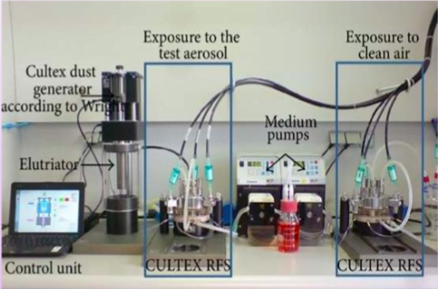
Cell culture (CULTEX)
- New experimental method for cultivation and exposure of cells of respiratory tract to air borne pollutants at air/liquid interface
- Enhanced efficiency of in-vitro studies
- Principle: Direct exposure of bronchial epithelial cells to complex mixtures
- Aim: To study factors influencing susceptibility of human bronchial epithelial cells
Procedure
- Bronchial epithelial cells washed with PBS
- Incubate with test drug for 24hrs
- Cells exposed to clean air/different concentrations of smoke for 1hr (Cell exposure unit)
- In-vivo models:
- activity in anesthetized guinea pigs (Konzett-Rössler method)
- Effect of arachidonic acid or PAF on respiratory function
- Histamine induced bronchospasm in GP
- Histamine induced bronchoconstriction in anesthetized GP: Body plethysmography and respiratory parameters
- Transgenic model
Evaluation in animal models
Choice of animal
Mouse: The most important advantage of the mouse model of asthma is the development of transgenic technology; there are many mouse-specific probes without particular genes which allow the study of genetic factors in the pathology of asthma.
- BALB/c mice displayed greater levels of airway reactivity to methacholine than C57BL/6 mice.
- Moreover, BALB/c mice exhibited higher numbers of mast cells in lung tissue when compared to C57BL/6.
- IL (Interleukin)-4, IL-5, IL-13, and CCL11 levels measured in whole-lung extracts were higher in BALB/c
Rats as animal model of asthma
- More serum and BAL for analysis
- Easily sensitised by ovalbumin, house dust mites extracts, Ascaris antigens – many reagents available for sensitisation
- Transgenic technology applications available.
- However, rat is a weak bronchoconstrictor.
- Rat model focussed mainly on inflammatory processes.
- Brown Norway strain – naturally atopic, more pronounced IgE and inflammatory response to allergen following sensitisation.
- Wistar rats can also be sensitised but comparative less response.
- Sprague Dawley and Fisher/Lewis rats do not develop an allergic reaction.
Guinea pig
- The guinea pig model was the first described model of asthma and contributed greatly to the development of corticosteroid and beta 2 receptor agonist therapies.
Broncho-spasmolytic activity in anesthetized GP (Konzett Rossler method):
- Bronchospasm decreases the volume of inspired air and increases the volume of excess air. Thus, the degree of bronchospasm can be quantified by recording the volume of excess air
- Evaluation of a drug’s bronchospasmolytic effect by measuring the volume of air, which is not taken up by the lungs after bronchospasm
- GP anesthetized with phenobarbitone.
- The trachea is cannulated with a 2 way cannula, one arm of which is connected to a respiratory pump and the other is connected to a pressure transducer.
- The animal is maintained by artificial respiration at 60 strokes per min, TV at 3 cu m/ 100g and inspiratory pressure is maintained at 90-120 mm of water.
- Test/control drug is administered i.v. or s.c and then broanchospasms are obtained at different time intervals by injecting with histamine/Ach/bradykinin/LT/PAF/substance P.
- The change in the pressure recorded by the transducer is compared between test and control.
- Results are expressed as percent inhibition of induced bronchospasm over the control agonistic responses.
- The ED50 value is calculated
Effect of arachidonic acid or PAF on respiratory function in vivo
- The test allows to evaluate the sites of action of drugs, which interfere with the mechanisms of broncho-constriction and thrombocytopenia
- Thromboxane causes bronchoconstriction and thrombocytopenia
- PGI2 causes ↓SBP and ↓DBP
- Aim: To study and compare:
- %inhibition or increase in bronchospasm
- BP reduction (measure magnitude and duration)
- Thrombocytopenia and hematocrit
Trachea → artificial respiration
jugular vein → test drug
carotid artery → blood withdrawal & transducer for BP measurement
- Record BP & changes in airflow
- multiple iv injections of same dose of arachidonic acid until two bronchospasms of equal intensity
- test compound administered iv spasmogen given again at: 2, 10, 20 and 30 min after test drug
Histamine induced bronchospasm in GP:
- GP are divided into test and control and administered respective drugs.
- Then the animals are placed in a histamine chamber and exposed to aerosolized histamine.
- Time required for asphyxia and convulsion are compared between test and control.
Histamine induced bronchoconstriction in anesthetized GP:
- GP anesthetized and the trachea, pleural cavity and jugular vein are cannulated.
- They are then placed in a plethysmograph in which flow in and out is recorded by a pressure transducer.
- The tracheal cannula is connected to the respiratory pump which maintains the respiration and measures the TV.
- The Trans-pulmonary pressure is measured by finding the difference in pressure between the pleura and tracheal cannula inlet.
- Also recorded is the Pulmonary resistance and Dynamic compliance by feeding the relevant values in the computer software.
- Test/control are injected in the IJV and this is f/b injecting histamine and changes in the 4 above mentioned parameters is compared between test and control.
Transgenic & Knockout models
- Plasmid mediated downregulation IL-5 in ovalbumin sensitized Brown Norway rats
- C57b1/6 Mice knockout model for IL-4
- IL-5Ra knockout model for IL-5
Clinical evaluation:
- Phase 1:
- Objectives: Determine the PK and PD. Determine the extent of systemic absorption of inhaled drugs.
- Subjects: Healthy volunteers.
- Phase 2:
- Design: Randomized, double blind, placebo controlled and or active controlled studies.
- Endpoints:
- B2 agonist: Cummulative dose response using FEV1 a/c/t control.
- Anti-inflammatory drugs: parallel group comparative studies are likely to be necessary comparing at least two, if not, more doses of the test drug with two doses of the comparator drug. (Lung function tests)
- Immunotherapy: bronchial provocation test or reduction of controller medication may be considered for efficacy analysis.
- Subjects: Known asthmatics with FEV1 between 50 and 80% on inhaled corticosteroids
- Phase 3:
- Reliever meds
- Design: parallel group, double blind, randomised and controlled. Efficacy may be shown in short-term trials of four-week duration.
- Comparator: The preferred option is a three-arm study where the new drug is compared with placebo and with a short-acting β2 agonist.
- Endpoint: Change in FEV1.
- Controller meds
- Design: randomised, double blind, parallel group, controlled clinical trials of at least six months duration
- Comparator: The established use of inhaled corticosteroids as first choice controller treatment for most patients makes these drugs the comparator of choice. A three-arm study including a comparison with placebo is strongly recommended in at least one pivotal clinical study, in order to ensure assay sensitivity.
- Endpoint: Lung functions and Symptoms based clinical endpoints.
- Specific immunotherapy
- Design parallel group, double blind, randomised, and controlled.
- Comparator: Placebo, since the immunotherapy is used as an add-on to existing treatments.
- Endpoint: Lung function, composite scores, number of exacerbations or reduced need for controller medication.
- Long term clinical safety: Long-term safety data from at least 1 year of treatment should be provided.
- Inhaled route: vocal cord myopathy, oral fungal infection or cataract formation.
