Glaucoma
- Glaucoma is the second leading cause of blindness in the world.
- Glaucoma is characterized by progressive damage to optic nerve associated with raised intraocular pressure (> 21 mm Hg).
- The term “ocular hypertension” is used for people with consistently raised intraocular pressure (IOP) without any associated optic nerve damage.
- Normal tension glaucoma: Optic disc changes despite normal IOP
- Primary Open Angle Glaucoma (POAG): Patency of trabecular meshwork affected
- Primary Angle Closure Glaucoma (PACG): Shallow ant. Chamber, narrow iridiocorneal angle
- Raised intraocular pressure which normally ranges from 10-21 mm of Hg is the most important and only modifiable risk factor for glaucoma and IOP-lowering medications comprise the primary treatment strategy.
- Rise in intraocular tension is either due to excessive production or due to less drainage of aqueous humor.
- Lowering of i.o.t. retards progression of optic nerve damage even in normal/low i.o.t. glaucoma
Pathophysiology
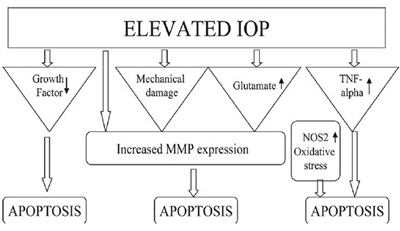
- ↑IOP – glaucomatous optic neuropathy involving retinal ganglion cell death d/t apoptosis.
- Characterized by changes in the optic disc and irreversible visual field defects.
- Optic disc changes: a) Neuroretinal rim thinning
b) Pallor &
c) Progressive optic disc cupping
- Visual field defects detected only after 40% of the axons are lost.
- Primary factors :
- ↑IOP
- Vascular dysregulation
- Secondary factors:
- Excitotoxic damage caused by glutamate or glycine released from injured neurons
- Oxidative damage caused by over-production of nitric oxide (NO) & ROS.
Major amount of aqueous (- 90%) drains through the trabecular route, while ~ I 0% fluid passes into the connective tissue spaces within the ciliary muscle- then via suprachoroidal into episcleral vessels (uveoscleral outflow).
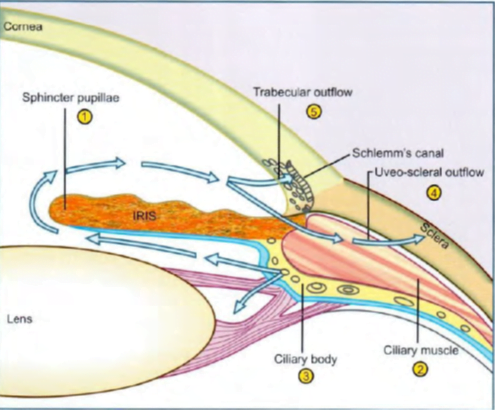
- Site of action of miotics in angle closure glaucoma: contraction of sphincter pupillae removes pupillary block and reverses obliteration of iridocorneal angle
- Site of action of miotics in open angle glaucoma: contraction of ciliary muscle pulls on scleral spur and improves trabecular patency
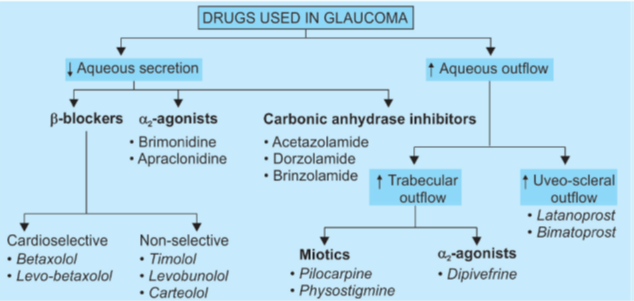
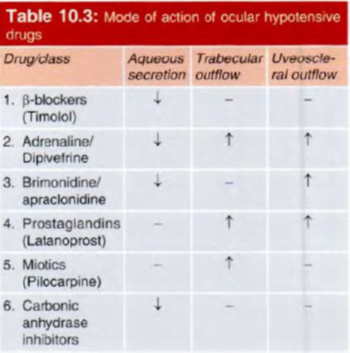
- Site of action of (a) beta blockers (b) a1 agonists (c) a2 agonists (d) carbonic anhydrase inhibitors: all reduce aqueous secretion by ciliary body
- Site of action of prostaglandins and adrenaline (alpha agonist action): increase uveoscleral outflow by altering permeability and/or pressure gradients
- Site of action of adrenaline (beta2 agonist action): possibly increases aqueous conductivity of trabecular filtering cells
Drugs useful in primary open angle glaucoma (POAG) are:
Ocular hypotensive drugs are used on a long-term basis and constitute the definitive treatment in majority of cases.
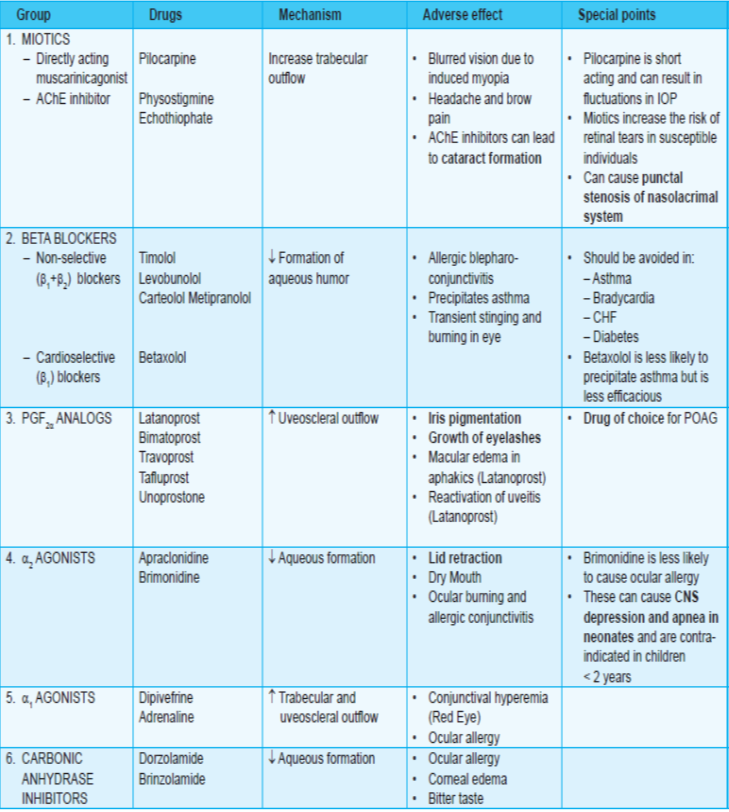
Prostaglandin analogs:
- PGF2α increases uveoscleral outflow.
- Latanoprost (i.o.t. reduction by 25-35%), bimatoprost and unoprostone are PGF2α derivatives useful in glaucoma.
- It reduces i.o.t. in normal pressure glaucoma also.
- good efficacy, once daily application and absence of systemic complicationsà drug of choice for POAG.
- Bimatoprost à s/e growth of eyelashes à can be utilized for treatment of hypotrichosis.
- Though ocular irritation and pain are relatively frequent, no systemic side effects are reported. Blurring of vision, increased iris pigmentation, thickening and darkening of eyelashes have occurred in some cases. Macular edema can develop during treatment with any PGF20 analogue, especially in aphakic patients; a watch should be kept to-detect it early.
- Travoprost – i.o.t. lowering efficacy and side effects are comparable to latanoprost.
β-blockers:
- among the first line drugs for POAG.
- Ciliary processes contain β2 (vasodilatory) and α2 (vasoconstrictor) receptors.
- Whenever vasodilation occurs, amount of blood reaching in the ciliary body increases resulting in excessive secretion of aqueous humor.
- Therefore, β-blockers and α agonists can decrease the secretion of aqueous.
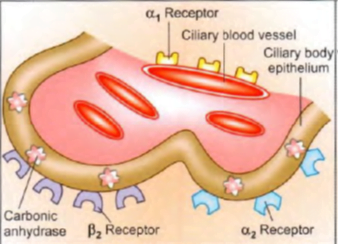
- α1 adrenoceptors constrict ciliary vessels and reduce aqueous production: are activated by adrenaline.
- α2 adrenoceptors located on ciliary epithelium reduce aqueous secretion: are activated by adrenaline/apraclonidine/brimonidine.
- β3 adrenoceptors located on ciliary epithelium enhance aqueous secretion via increased cAMP. Their blockade by timolol/betaxolol reduces secretion.
- Carbonic anhydrase present within ciliary epithelial cells generates HCO3-; ion which is secreted into aqueous humor. Acetazolamide/dorzolamide inhibit it and reduce
aqueous secretion.
- Timolol, betaxolol, levobetaxolol, levobunolol, carteolol and metipranolol have been approved for use in glaucoma.
- Levobunolol is longest acting whereas betaxolol is cardioselective (therefore less efficacious but safe in asthmatics) β-blocker.
- 20-35% fall in i.o.t.
- Ocular side effects of beta blockers à generally mild and infrequent- stinging, redness and dryness of eye, corneal hypoesthesia, allergic blepharoconjunctivitis and blurred vision.
- Systemic adverse effects à major limitations à occur due to absorption through nasolacrimal duct à life threatening bronchospasm in asthmatic and COPD patients.
Bradycardia, accentuation of heart block and CHF are likely to occur especially in the elderly.
Systemic adverse effects can be minimized by applying mild pressure on the inner canthus of the eye for about 5 min. after instilling the eye drop to prevent entry of the drug into nasolacrimal duct from where it is mainly absorbed.
Betaxolol à beta1 selective blocker àless bronchopulmonary and probably less cardiac, central and metabolic side effects.
In addition, it appears to exert a protective effect on retinal neurons independent of i.o.t. lowering, by blocking some Ca2+ channels and reducing Na+/Ca2 influx.
Advantages of topical beta blockers over miotics
- No change in pupil size: no diminution of vision in dim light and in patients with cataract
- No induced myopia which is especially troublesome in young patients
- No headache/brow pain due to persistent spasm of iris and ciliary muscles
- No fluctuations in i.o.t. as occur with pilocarpine drops
- Convenient twice/once daily application sufficient
α-Agonists:
- Dipivefrine (prodrug of adrenaline) and adrenaline act by increasing trabecular outflow whereas
- apraclonidine (lowers i.o.t. by ~25%.)and brimonidine (selective a2 agonists) act by decreasing aqueous secretion.
- Apraclonidine can cause lid retraction, itching, lid dermatitis, follicular conjunctivitis, mydriasis, dryness of mouth and nose
- brimonidine (lowers i.o.t. by 20- 27%)is associated with anterior uveitis. Allergic conjunctivitis and other ocular side effects are similar to but less frequent than with apraclonidine.
- Both of these can cause drowsiness.
- Dipivefrine can cause cystoid macular edema in aphakics.
Carbonic anhydrase inhibitors:
- Acetazolamide (oral), brinzolamide and dorzolamide (both topical) act by decreasing the secretion of aqueous humor
- Oral treatment with acetazolamide (0.25 g 6-12 hourly) reduces aqueous formation by limiting generation of bicarbonate ion in the ciliary epithelium
- used to supplement ocular hypotensive drugs for short-term indications like angle closure, before and after ocular surgery/laser therapy
- Dorzolamide – lowers i.o.t. by ~20%; Ocular stinging, burning, itching, corneal edema and bitter taste are the usual side effects.
Miotics:
- Pilocarpine (directly acting cholinomimetic) and physostigmine (indirectly acting cholinomimetic) increase aqueous outflow by causing miosis.
- Pilocarpine is short acting, therefore requires frequent daily dosing.
- Demacarium and ecothiophate (both are long acting cholinomimetics) à rarely used à accelerate cataract development.
For closed-angle glaucoma,
- occurs in individuals with a narrow iridocorneal angle and shallow anterior chamber. The i.o.t. remains normal until an attack is precipitated, usually by mydriasis
- The i.o.t. rises rapidly to very high values (40- 60 mmHg).
- Vigorous therapy employing several measures to reduce i.o.t. is instituted.
- Hypertonic mannitol (20%) 1.5- 2 g/kg or glycerol (10%):
infused i.v. decongest the eye by osmotic action.
- Acetazolamide: 0.5 g i.v. followed by oral twice daily is started concurrently.
- Miotic: Once the i.o.t. starts falling due to the above i.v. therapy, pilocarpine 1-4% is instilled every 10 min initially and then at longer intervals.
Pupillary block is removed and iridocorneal angle is freed
- Topical beta blocker: Timolol 0.5% is instilled 12 hourly in addition.
- Apraclonidine ( 1 %)/latanoprost 0.005% instillation may be added.
- Drugs are used only to terminate the attack of angle closure glaucoma.
- definitive treatment is surgery à Laser peripheral iridotomy or surgical peripheral iridectomy.
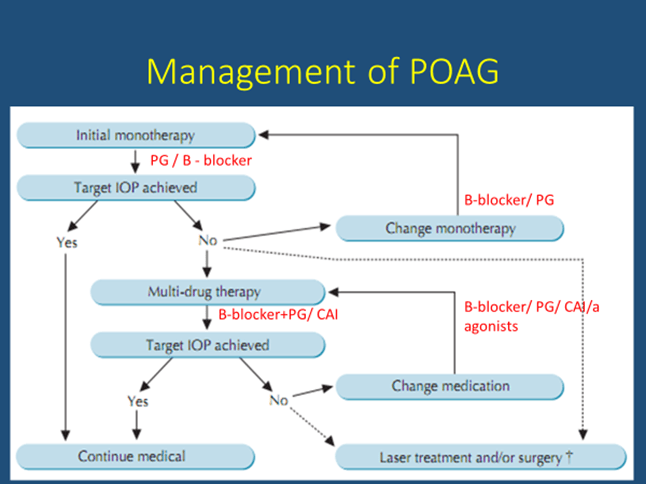
Why new drugs?
- Neural damage irreversible – need for neuroprotective agents
- Patients with asthma, bradycardia, cataract, allergy to sulfa drugs or topical brimonidine – not much options left other than SX
- Need for preservative free drugs
- Benzalkonium – punctate / ulcerative keratopathy
- Thiomersal – hypersensitivity
- Drugs for newer drug delivery systems
- lanoprostene bunod
- FDA approved in November 2017
- releases nitric oxideà relax the trabecular meshwork to increase drainage of aqueous humor and improve aqueous humor outflow.
- first nitric oxide-containing medication for glaucoma treatment worldwide.
- Clinical trials à comparing lanoprostene to latanoprost and timolol à greater eye pressure lowering compared to latanoprost and timolol.
- Side effects à similar to that of prostaglandin analogues
- Common side effects à redness of the conjunctiva, eyelash growth, eye irritation, and eye pain at the time of instilling the medication
- Netarsudil
- rho-associated protein kinase inhibitors, or ROCK inhibitors.
- approved by the FDA in December 2017
- ROCK à control cellular structures involved in cell shape, motility, contractility, proliferation, and apoptosis.
- ROCK inhibitors à lower eye pressure by relaxing the trabecular meshwork and connection passageway, called Schlemm’s canal.
- This canal drains the aqueous humor from the anterior chamber of the eye into the veins draining the eyeball.
- this decreases the resistance to drainage or outflow in the conventional outflow pathway, thus lowering eye pressure.
- some preliminary laboratory à role in promoting survival of the retinal ganglion cells à mechanism has not yet proven
- Clinical trials comparing Netarsudil to latanoprost and timolol à lowers eye pressure to a similar degree.
- The most frequent side effects include eye redness, small bleeds in the conjunctiva, and corneal changes. Rhopressa is dosed twice daily
- Ripasudil – Rho-associated protein kinase (ROCK) inhibitor – Approved in Japan in September 2014 and in commercial use in Japan
- Netarsudil + Latanoprost – ROCK and norepinephrine transporter inhibitor + prostaglandin analogue – Phase III trials completed
- Trabodenoson – Adenosine receptor agonists –
- Adenosine receptor agonists à stimulate secretion of matrix metalloproteinases (MMPs) in the endothelial cells lining the trabecular meshwork.
- This causes cell volume shrinkage and extracellular matrix remodeling à facilitates conventional aqueous outflow.[
- Trabodenoson à Phase III clinical trial completed
- Phase II trials demonstrated a median IOP reduction of −6.5 mmg ± 2.5 mmHg at 500 mcg dose by day 28 of trial.
- Prostanoid receptor agonists
- latanoprost and travoprost, act on prostanoid prostaglandin F receptor (FP receptor), a receptor for Prostaglandin F2 alpha (PGF2α).
- Bimatoprost is a synthetic PGF2α mimetic, termed prostamide F2α.
- These drugs enhance aqueous outflow through the uveoscleral pathway and cause IOP lowering.
- Recently, EP2, EP3 receptors à DE‑117 à an EP2 agonist.
- cause relaxation of endothelial cells in the Schlemm’s canal, facilitating uveoscleral outflow.
- also increase conventional outflow by acting on the trabecular meshwork, decreasing cell contractility and collagen deposition
- Taprenepag isopropyl is an EP2 agonist. Results from Phase II showed that taprenepag was comparable to latanoprost 0.005% in IOP reduction
- Small-interfering RNA – Bamosiran
- Bamosiran is a naked double‑stranded small‑interferingRNA.
- Acts through specific gene silencing and causes beta‑2 adrenergic receptor blockade, thereby decreasing aqueous production by the ciliary body.
- After topical administration, the drug is rapidly distributed in the anterior chamber.
- It is preferentially taken up by the ciliary body cells; hence, undesirable beta‑receptor blockade in bronchioles and alveoli is limited
- Phase IIb of a clinical trial which compared escalating concentrations of Bamosiran (0.375%– 1.5%) qd with 0.5% timolol bd, found 1.125% Bamosiran to be non-inferior to timolol 0.5%, in patients with a baseline IOP >25 mmHg.
- Preservative‑free, Newer Preservatives, and Self‑preserved IOP‑Lowering Medications
- Glaucoma patients have a high prevalence of ocular surface diseases. These have known to impact the quality of life and adherence to IOP‑lowering medication.
- Many of these symptoms can be drug induced or due to the commonly used preservative benzalkonium chloride (BAK).
- BAK has been shown to exacerbate ocular surface conditions such as meibomian gland dysfunction, chronic conjunctival inflammation, keratitis, and causes tear film instability.
- As per pharmacopeia recommendations, it is mandatory to have a preservative for microbial static or cidal action, once bottle is opened.
- Hence, the need for newer, less toxic preservatives.
- Purite is a stabilized oxychloro complex (SOC).
- It has a broad‑spectrum antimicrobial activity even at low concentrations (0.005%).
- Studies have shown that decreasing brimonidine concentration from 0.2% to 0.15% and replacing BAK with SOC improve drug tolerance, especially in irritated eyes.
- Polyquad (Polyquaternium‑1) à for preserving some prostaglandin analogs (Travatan, Travoprost 0.004%, Alcon labs) and some tear substitutes. à better tolerated than BAK, it does have some proinflammatory and cytotoxic effects on corneal epithelial cells.
- Neuroprotection
- glaucoma à progression and RGC loss can occur despite IOP lowering.
- need to find mechanisms and drugs that retard neuronal apoptosis and degeneration.
- Memantine à selective N‑methyl‑d‑aspartate receptor antagonist à potential to prevent glutamate‑induced excitotoxicity of RGCs. A randomized double‑masked placebo‑controlled clinical trial which was studying the neuroprotective effects of memantine failed to meet its primary endpoint
- Low‑pressure glaucoma treatment study was a randomized trial comparing the efficacy of 0.2% brimonidine tartrate with 0.5% timolol maleate in preserving visual function in patients with low‑pressure glaucoma. A lower rate of visual field progression was seen with brimonidine‑treated patients compared to those receiving timolol monotherapy, despite achieving a similar mean IOP reduction. This was attributed to neuroprotective effect of brimonidine.
- Novel Drug Delivery Systems
- Nanoparticle-based topical formulations
- Nanoparticle à 10–1000 nm serves as vehicles for drug delivery.
- bioinert, biodegradable, and mucoadhesive polymers which aid corneal penetration of the drug.
- In vivo studies of carbonic anhydrase inhibitors, brimonidine, pilocarpine, using this delivery system has shown better drug permeability and stability compared to their commercially available counterparts.
- Contact lens-based drug delivery
- Silicone hydrogel soft contact lenses loaded with nanoparticles containing timolol have been found to elute the drug for more than a month in animal models
- One big drawback while using hydrogels is that the drug elutes very quickly from the highly hydrated polymer networks as most IOP‑lowering medications are water soluble.
- Ocular inserts à A randomized control study compared the IOP‑lowering efficacy of topical bimatoprost ocular insert to twice daily timolol in POAG and OHT patients treated for 6 months.
- A clinically relevant sustained IOP reduction of 4–6 mmHg was achieved with the insert group. However, daily application of timolol produced IOP reduction of 1.5 mmHg more than the insert.
- Comfort profile and tolerability were found to be similar to topical bimatoprost 0.003%.
- Travoprost punctal plug à rod‑shaped polyethylene glycol‑based hydrogel punctum plug encapsulating the active drug.
- It is placed in the vertical portion of the superior or inferior canaliculus.
- A study investigating that its safety and efficacy in an Asian population found IOP reduction of 24% (day 10) and 15. 6% (day 30), respectively.
- Bimatoprost sustained‑release (SR) implantsà biodegradable implants which help in sustained drug delivery over 4–6 months.
- It is administered intracamerally through a prefilled applicator. Six‑month results from Phase I/II clinical trial which compared topical bimatoprost 0.003% to intracameral administration of 6, 10, and 15 mcg of the drug showed favorable results
- Gene Therapy in Glaucoma
- use of targeted gene therapy.
- Both viral and non-viral vectors are used to deliver genes to target tissue of interest such as trabecular meshwork, ciliary epithelium, ciliary body, and RGC.
- Gene therapy can be used to delete, replace/inactivate an aberrant gene, or introduce a new gene which helps in targeted therapeutic protein expression.
- Downregulation of MMP1 expression in the trabecular meshwork is postulated to play a role in the pathophysiology of steroid‑induced ocular hypertension.
- Animal studies have shown that intracameral injection of adenovius carrying the recombinant MMP1 resulted in reversal of corticosteroid‑induced OHT.
- Loss of cyclooxygenase (COX‑2) in nonpigmented ciliary epithelial cells has been implicated in the pathophysiology of POAG.
- Animal studies have demonstrated that intracameral delivery of lentivirus vectors expressing COX‑2 and FP resulted in transduction of trabecular meshwork and ciliary epithelium and ultimately IOP reduction.
- Adeno‑associated viral vectors (AAV) have been used to deliver antiapoptotic genes to the retina in rodent glaucoma models.
- Intravitreal injections of AAV expressing brain‑derived neurotrophic factor have resulted in RGC survival for a month in rodent models with induced OHT
