Inflammatory Bowel Disease
- includes Crohn disease and ulcerative colitis, is a relapsing and remitting condition characterized by chronic inflammation at various sites in the GI tract, which results in diarrhea and abdominal pain.
Pathophysiology
- Inflammation results from a cell-mediated immune response in the GI mucosa.
- precise etiology à unknown, but evidence à normal intestinal flora trigger an abnormal immune reaction in patients with a multifactorial genetic predisposition (perhaps involving abnormal epithelial barriers and mucosal immune defenses).
- No specific environmental, dietary, or infectious causes have been identified.
- The immune reaction involves the release of inflammatory mediators, including cytokines, interleukins, and TNF.
Epidemiology
- Affects all ages but usually begins before age 30, with peak incidence from 14 to 24.
- may have a second smaller peak between ages 50 and 70
- Both sexes are equally affected.
- First-degree relatives of patients with IBD have a 4- to 20-fold increased risk; their absolute risk may be as high as 7%.
- Cigarette smoking seems to contribute to development or exacerbation of Crohn disease but decreases risk of ulcerative colitis.
- Appendectomy done to treat appendicitis also appears to lower the risk of ulcerative colitis.
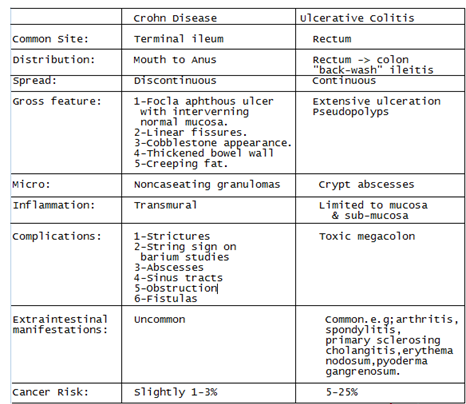
Extraintestinal Manifestations
- usually parallel (ie, wax and wane with) IBD flare-ups: These disorders include peripheral arthritis, episcleritis, aphthous stomatitis, and erythema nodosum. Arthritis tends to involve large joints
- appear independently of IBD activity: These disorders include ankylosing spondylitis, sacroiliitis, uveitis, pyoderma gangrenosum, and primary sclerosing cholangitis
- consequences of disrupted bowel physiology à Malabsorption à cause deficiencies of fat-soluble vitamins, vitamin B12, or minerals, resulting in anemia, hypocalcemia, hypomagnesemia, clotting disorders, and bone demineralization. In children, malabsorption retards growth and development
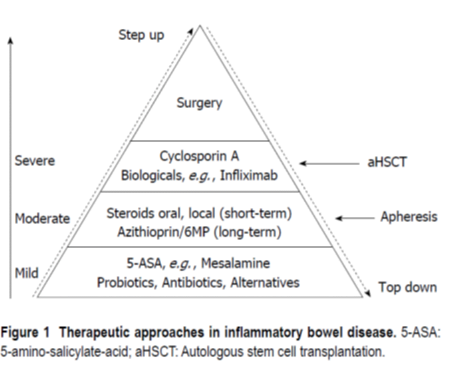
Overview of Stepwise Therapy
- first step à usually aminosalicylates à several different aminosalicylates à but none have been consistently demonstrated to be superior to the others for all patients.
- greater efficacy for the treatment of ulcerative colitis than for Crohn disease. For Crohn disease, metronidazole or ciprofloxacin is occasionally used, particularly for perianal disease or an inflammatory mass.
- If the patient à fails to respond to an adequate dose of aminosalicylates,
- second step is often corticosteroids à provide rapid relief of symptoms and a significant decrease in inflammation.
- most common range for moderate flares of IBD is oral prednisone at 10-40 mg/day;
- for more severe flares, the higher end of the range is used (occasionally doses up to 60 mg/day).
- Once a clinical response is seen, the dose is tapered.
- Inability to taper down the steroids without recurrence of symptoms should trigger discussion regarding the use of alternative drugs (immunomodulators or anti-TNF therapy).
- immune-modifying agents à step III drugs à used if corticosteroids fail or are required for prolonged periods.
- Anti-TNF monoclonal antibody à effective in both Crohn disease and ulcerative colitis
5-Aminosalicylic Acid (5-ASA,Mesalamine)
- 5-ASA à blocks production of prostaglandins and leukotrienes à beneficial effects on the inflammatory cascade.
- Because 5-ASA is active only intraluminally and is rapidly absorbed by the proximal small bowel, it must be formulated for delayed absorption when given orally.
- Sulfasalazine, the original agent in this class, à delays absorption by complexing 5-ASA with a sulfa moiety, sulfapyridine.
- The complex is cleaved by bacterial flora in the lower ileum and colon, releasing the 5-ASA.
- The sulfa moiety, however, causes numerous adverse effects (eg, nausea, dyspepsia, headache), interferes with folate (folic acid) absorption, and occasionally causes serious adverse reactions (eg, hemolytic anemia or agranulocytosis and, rarely, hepatitis, pneumonitis, or myocarditis).
- Reversible decreases in sperm count and motility occur in up to 80% of men.
- If used, sulfasalazine should be given with food, initially in a low dosage (eg, 0.5 g orally twice a day) and the dose and frequency gradually increased over several days to 1 to 1.5 g 4 times a day.
- Patients should take daily folate supplements (1 mg orally) and have CBC and liver tests every 6 to 12 months.
- Acute interstitial nephritis secondary to mesalamine occurs rarely; periodic monitoring of renal function is advisable because most cases are reversible if recognized early.
- Drugs that complex 5-ASA with other vehicles seem almost equally effective but have fewer adverse effects.
- Olsalazine (a 5-ASA dimer) and balsalazide (5-ASA conjugated to an inactive compound) are cleaved by bacterial azoreductases (as is sulfasalazine).
- These drugs are activated mainly in the colon and are less effective for proximal small-bowel disease.
- Olsalazine dosage is 1000 mg orally twice a day, and balsalazide is 2.25 g orally 3 times a day.
- Olsalazine sometimes causes diarrhea, especially in patients with pancolitis. This problem is minimized by gradual escalation of dose and administration with meals.
- Other formulations of 5-ASA use delayed-release and/or extended-release coatings. All of these formulations of 5-ASA are therapeutically roughly equivalent.
- 5-ASA is also available as a suppository (500 or 1000 mg at bedtime or twice a day) or enema (4 g at bedtime or twice a day) for proctitis and left-sided colon disease.
Corticosteroids
- useful for acute flare-ups of most forms of IBD when 5-ASA compounds are inadequate.
- However, corticosteroids are not appropriate for maintenance.
- IV hydrocortisone 300 mg/day or methylprednisolone 16 to 20 mg 3 times a day is used for severe disease;
- oral prednisone or prednisolone 40 to 60 mg once/day may be used for moderate disease.
- Treatment is continued until symptoms remit (usually 7 to 28 days) and then tapered by 5 to 10 mg weekly to 20 mg once/day.
- Treatment is then further tapered by 2.5 to 5 mg weekly depending upon clinical response, while instituting maintenance therapy with 5-ASA or immunomodulators.
- Adverse effects of short-term corticosteroids in high doses include hyperglycemia, hypertension, insomnia, hyperactivity, and acute psychotic episodes.
- Budesonide is a corticosteroid with a high (> 90%) first-pass liver metabolism; thus, oral administration may have a significant effect on GI tract disease but minimal adrenal suppression.
- Oral budesonide has fewer adverse effects than prednisolone but is not as rapidly effective and is typically used for less severe disease.
- All patients started on corticosteroids (including budesonide) should be given oral vitamin D 400 to 800 units/day and calcium 1200 mg/day.
Immunomodulating Drugs
- The antimetabolites azathioprine, 6-mercaptopurine, and methotrexate are also used in combination therapy with biologic agents.
Azathioprine and 6-mercaptopurine
- Azathioprine and its metabolite 6-mercaptopurine inhibit T-cell function and may induce T-cell apoptosis.
- effective long-term and may diminish corticosteroid requirements and maintain remission for years.
- often require 1 to 3 months to produce clinical benefits, so corticosteroids cannot be completely withdrawn until at least the 2nd month.
- Dosage of azathioprine is usually 2.5 to 3.0 mg/kg orally once/day and
- 6-mercaptopurine is 1 to 1.5 mg/kg orally once/day
- The most common adverse effects are nausea, vomiting, and malaise. Signs of bone marrow suppression must be monitored with regular WBC count (biweekly for 1 month, then every 1 to 2 months).
- Pancreatitis or high fever occurs in about 3 to 5% of patients; either is an absolute contraindication to rechallenge.
- Hepatotoxicity is rarer and can be screened by blood tests every 6 to 12 months. These drugs are associated with increased risk of lymphoma and nonmelanoma skin cancers.
Methotrexate
- 15 to 25 mg orally or sc weekly à corticosteroid-refractory or corticosteroid-dependent Crohn disease, even those who have not responded to azathioprine or 6-mercaptopurine.
- Adverse effects include nausea, vomiting, and asymptomatic liver function test abnormalities.
- Folate 1 mg orally once/day may diminish some of the adverse effects.
- Both women and men taking methotrexate should ensure the female partner uses an effective contraceptive method such as an intrauterine device, a contraceptive implant, or an oral contraceptive.
- Less effective methods of contraception, such as condoms, spermicides, diaphragms, cervical caps, and periodic abstinence, should be discouraged.
- Additionally, women and perhaps men should stop methotrexate for at least 3 months before trying to conceive.
- Monthly CBCs and liver function tests with albumin should be done for the first 3 months of therapy then every 8 to 12 weeks during therapy.
- Alcohol use, hepatitis B and C, obesity, diabetes, and possibly psoriasis are risk factors for hepatotoxicity.
Cyclosporine and tacrolimus
- blocks lymphocyte activation, may benefit patients with severe ulcerative colitis unresponsive to corticosteroids and biologic agents and who may otherwise require colectomy.
- Its only well-documented use in Crohn disease is for patients with refractory fistulas or pyoderma.
- Initial dose is 2 to 4 mg/kg IV in continuous infusion over 24 hours; responders are converted to an oral dose of 6 to 8 mg/kg once/day with early introduction of azathioprine or 6-mercaptopurine.
- Long-term use (> 6 months) is contraindicated by multiple adverse effects (eg, renal toxicity, seizures, opportunistic infections, hypertension, neuropathy).
- Tacrolimus, an immunosuppressant also used in transplant patients, seems as effective as cyclosporine and may be considered for use in patients with severe or refractory ulcerative colitis who do not require hospitalization.
Biologic Agents
Anti-TNF drugs
- Infliximab, certolizumab, adalimumab, and golimumab are antibodies to tumor necrosis factor (TNF).
- useful in Crohn disease, particularly in preventing or retarding postoperative recurrence and are beneficial in ulcerative colitis for refractory or corticosteroid-dependent disease.
- Infliximab à single IV infusion of 5 mg/kg over 2 hours. It is followed by repeat infusions at weeks 2 and 6. Subsequently, it is given every 8 weeks.
- Adalimumab is approved for Crohn disease and ulcerative colitis. It is given with an initial loading dose of 160 mg sc and then 80 mg sc at week 2. After that dose, 40 mg sc is given every 2 weeks. The dose should be adjusted to achieve a therapeutic serum level of > 7.5 mcg/mL. Patients who are intolerant or who have lost their initial response to infliximab may respond to adalimumab therapy.
- Certolizumab is approved for Crohn disease. It is given as 400 mg sc every 2 weeks for three doses and then every 4 weeks for maintenance. Patients who are intolerant of or who have lost their initial response to infliximab may respond to certolizumab. The accepted therapeutic serum level is > 20 mcg/mL.
- Golimumab is approved for use in patients with ulcerative colitis. It is given with an initial loading dose of 200 mg sc and then 100 mg at week 2. After that dose, 100 mg is given every 4 weeks. Patients who are intolerant or who have lost their initial response to infliximab may respond to golimumab therapy.
- Monotherapy with anti-TNF agents is clearly effective for both induction and maintenance of remission, but some studies suggest better results when anti-TNF agents are initiated in combination with a thiopurine (eg, azathioprine) or methotrexate.
- Adverse effects during infusion (infusion reaction) include immediate hypersensitivity reactions (eg, rash, itching, sometimes anaphylactoid reactions), fever, chills, headache, and nausea. Delayed hypersensitivity reactions have also occurred.
- Anti-TNF drugs given subcutaneously (eg, adalimumab) do not cause infusion reactions, although they may cause local erythema, pain, and itching (injection site reaction).
- anti-TNF use, à contraindicated when uncontrolled bacterial infection is present.
- Also, TB and hepatitis B reactivation has been attributed to anti-TNF drugs; therefore, screening for latent TB (with PPDs and/or interferon-gamma release assay) and for hepatitis B is required before therapy.
- Lymphoma, demyelinating disease, and liver and hematologic toxicity are other potential concerns with anti-TNF antibody treatment.
Other biologic agents
- Vedolizumab and natalizumab are antibodies to leukocyte adhesion molecules.
- The benefits of natalizumab for patients with Crohn’s disease should be carefully weighed against the potential risk for PML
- Vedolizumab has been approved for moderate to severe ulcerative colitis and Crohn disease. The recommended dose of IV vedolizumab is 300 mg at 0, 2, and 6 weeks and then every 8 weeks. Its effect is believed to be limited to the gut, making it safer than natalizumab, which is used only as a 2nd-line drug through a restricted-prescribing program for the most refractory cases of Crohn disease.
- Ustekinumab, is a fully human monoclonal antibody directedagainst the p40 subunit of interleukin IL-12 and IL-23, is approved for patients with moderate to severe Crohn disease who have failed conventional therapy.
The initial loading dose is a single IV dose based on weight:
- < 55 kg: 260 mg
- 55 to 85 kg: 390 mg
- > 85 kg: 520 mg
- After the loading dose, patients are given a maintenance dose of 90 mg sc every 8 weeks. The accepted therapeutic serum level is > 5 mcg/mL.
Brazikumab
Selective blockage of IL-23 may increase safety by allowing the normal IL-12-mediated Th1 response required in the immune response to intracellular pathogens while conferring the same efficacy as with p40 antibodies
intravenous fully human IgG2 monoclonal antibody that selectively binds the p19 subunit on IL-23 with no impact on IL-12
Risankizumab
Risankizumab is also an intravenous humanized IL-23p19 inhibitor
- Other anticytokine, anti-integrin, small-molecule agents, and growth factors are under investigation, as is leukopheresis therapy to deplete activated immunocytes.
Small-molecule agents
- Small-molecule agents are drugs with molecular weight < 1 kilodalton. They are given orally and lack the immunogenicity associated with monoclonal antibodies.
- Tofacitinib is a small-molecule agent that inhibits Janus kinase 1–3 and is approved for adult patients with moderate to severe ulcerative colitis.
- Potential adverse effects include elevated cholesterol levels, diarrhea, headache, shingles (herpes zoster), augmented blood creatine phosphokinase, nasopharyngitis, rash, and upper respiratory tract infection. Other uncommon adverse effects include cancer and opportunistic infections. In addition, the FDA recently warned of an increased risk of fatal pulmonary embolism and death in patients with rheumatoid arthritis.
Filgotinib
Filgotinib is an oral second-generation selective JAK-1 inhibitor with 30-fold selectivity for JAK1– over JAK 2– dependent signaling, and 50 times selectivity for JAK 1 over JAK3
Upadacitinib is an oral selective JAK 1 inhibitor.
Antibiotics and Probiotics
- Antibiotics
- Antibiotics may be helpful in Crohn disease but are of limited use in ulcerative colitis, except in toxic colitis.
- Metronidazole 500 to 750 mg orally 3 times a day for 4 to 8 weeks may control mild Crohn disease and help heal fistulas. However, adverse effects (particularly neurotoxicity) often preclude completion of treatment.
- Ciprofloxacin 500 to 750 mg orally twice a day may prove less toxic. Many experts recommend metronidazole and ciprofloxacin in combination.
- Rifaximin, a nonabsorbable antibiotic, at a dose of 200 mg orally 3 times a day or 800 mg orally twice a day may also be beneficial as treatment for active Crohn disease.
Probiotics
- Various nonpathogenic microorganisms (eg, commensal Escherichia coli, Lactobacillus species, Saccharomyces) given daily serve as probiotics and may be effective in preventing pouchitis, but other therapeutic roles have yet to be clearly defined.
