Liposomal drug delivery system
- Advances in combinatorial chemistry have led to the discovery of a wide number of new chemical entities (NCE) that have a potential therapeutic action on the biological systems
- But most of the NCEs being discovered provide a challenge because of their physicochemical properties like poor solubility and permeability.
- Various carriers like nanoparticles, microparticles, polysaccharides, lectins and liposomes can be used to target the drug to a specific site
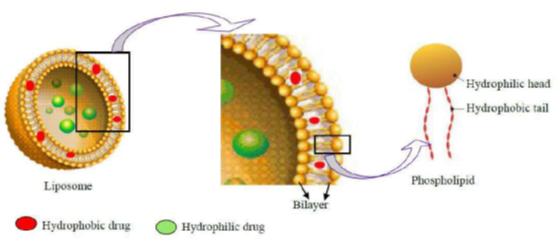
- Liposomes à act as a carrier for a variety of drugs, having a potential therapeutic action.
- Liposomes are colloidal carriers, having a size range of 0.01 – 5.0 μm in diameter.
- These are bi-layered vesicles that are formed when phospholipids are hydrated in excess of aqueous medium.
- advantage à encapsulating hydrophilic as well as hydrophobic drugs and targeting them to the required diseased site in the body
- Various therapeutic agents like anticancer drugs, vaccines, antimicrobials, genetic materials,
proteins and macromolecules can be encapsulated within the bilayered vesicles
MECHANISM OF LIPOSOME FORMATION:
- The basic part of liposome is formed by phospholipids, which are amphiphilic molecules (having a hydrophilic head and hydrophobic tail).
- hydrophilic part à phosphoric acid bound to a water-soluble molecule
- hydrophobic part à consists of two fatty acid chains with 10 – 24 carbon atoms and 0 – 6 double bonds in each chain
- When these phospholipids are dispersed in aqueous medium, they form lamellar sheets by organizing in such a way that,
- the polar head group faces outwards to the aqueous region while
- the fatty acid groups face each other and finally form spherical/ vesicle like structures called as liposomes.
- When phospholipids are hydrated in water, along with the input of energy like sonication, shaking, heating, homogenization, etc. it is the hydrophilic/ hydrophobic interactions between lipid – lipid, lipid – water molecules that lead to the formation of bilayered vesicles in order to achieve a thermodynamic equilibrium in the aqueous phase
- The reasons for bilayered formation include:
- The unfavorable interactions created between hydrophilic and hydrophobic phase can be minimized by folding into closed concentric vesicles.
- Large bilayered vesicle formation promotes the reduction of large free energy difference present between the hydrophilic and hydrophobic environment.
- Maximum stability to supramolecular self assembled structure can be attained by forming into vesicles.
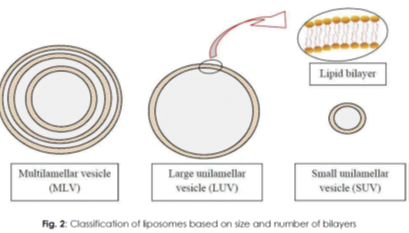
CLASSIFICATION OF LIPOSOMES:
classified based on their size, number of bilayers, composition and method of preparation.
- Multilamellar vesicles (MLV),
- large unilamellar vesicles (LUV) and
- small unilamellar vesicles (SUV)
Based on composition, they are classified as
- conventional liposomes (CL),
- pH-sensitive liposomes,
- cationic liposomes,
- long circulating liposomes (LCL) and immuno-liposomes.
Multilamellar vesicles (MLV)
- MLV have a size greater than 0.1 μm and consist of two or more bilayers. T
- They are mechanically stable on long storage.
- Due to the large size, they are cleared rapidly by the reticulo-endithelial system (RES) cells and hence can be useful for targeting the organs of RES
- MLV have a moderate trapped volume, i.e., amount of aqueous volume to lipid ratio.
Large unilamellar vesicles (LUV)
- This class of liposomes consists of a single bilayer and has a size greater than 0.1 μm.
- Have higher encapsulation efficiency, since they can hold a large volume of solution in their cavity
- They have high trapped volume and can be useful for encapsulating hydrophilic drugs.
- Advantage à less amount of lipid is required for encapsulating large quantity of drug.
- Similar to MLV, they are rapidly cleared by RES cells, due to their larger size
Small unilamellar vesicles (SUV)
- SUV are smaller in size (less than 0.1 μm) when compared to MLV and LUV, and have a single bilayer.
- have a low entrapped aqueous volume to lipid ratio and characterized by having long circulation half life.
- SUV can also be achieved by passing MLV through a narrow orifice under high pressure.
CHARACTERIZATION OF LIPOSOMES:
Size and size distribution
- When liposomes are intended for inhalation or parenteral administration, the size distribution is of primary consideration, since it influences the in vivo fate of liposomes along with the encapsulated drug molecules
- Various techniques of determining the size of the vesicles include microscopy (optical microscopy, negative stain transmission electron microscopy, diffraction and scattering techniques
Percent drug encapsulation
- The amount of drug encapsulated/ entrapped in liposome vesicle is given by percent drug encapsulation.
- Column chromatography can be used to estimate the percent drug encapsulation of liposomes
Surface charge
- Since the charge on the liposome surface plays a key role in the in vivo disposition, it is essential to know the surface charge on the vesicle surface.
- Two methods namely, free-flow electrophoresis and zeta potential measurement can be used to estimate the surface charge of the vesicle.
Phospholipid identification and assay
- The chemical components of liposomes must be analyzed prior to and after the preparation
- thin layer chromatography can be used to estimate the phospholipid concentration in the liposomal formulation.
- A spectrophotometric method to quantify total phosphorous
- Gas liquid chromatography techniques can be used to determine the cholesterol concentration
STABILITY OF LIPOSOMES:
- A well-designed stability study includes the evaluation of its physical, chemical and microbial parameters along with the assurance of product’s integrity throughout its storage period.
Physical stability
- Liposomes are bi-layered vesicles that are formed when phospholipids are hydrated in water.
- The vesicles obtained during this process are of different sizes.
- During its storage, the vesicles tend to aggregate and increase in size to attain thermodynamically favorable state.
- During storage, drug leakage from the vesicles can occur due to fusion and breaking of vesicles, which deteriorates the physical stability of the liposomal drug product.
- In order to monitor this à techniques à light scattering and electron microscopy à (morphology) and size of the vesicles.
Chemical stability
- Phospholipids are chemically unsaturated fatty acids that are prone to oxidation and hydrolysis, which may alter the stability of the drug product.
- Along with this, pH, ionic strength, solvent system and buffered species also play a major role in maintaining a liposomal formulation.
- prevented from oxidative degradation by protecting them from light, by adding anti-oxidants such as alpha – tocopherol or butylated hydroxyl toluene (BHT), or by adding EDTA to remove trace heavy metals
- Hydrolysis of the ester bond at carbon position of the glycerol moiety of phospholipids leads to the formation of lyso-phosphatidylcholine (lysoPC), which enhances the permeability of the
- liposomal contents.
- Hence, it becomes necessary to control the limit of lysoPC within the liposomal drug product.
- This can be achieved by formulating liposomes with phosphatidylcholine free from lysoPC
THERAPEUTIC APPLICATIONS OF LIPOSOMES:
- provide a superior therapeutic efficacy and safety in comparison to existing formulations.
Site-avoidance delivery
- The cytotoxicity of anti-cancer drugs to normal tissues can be attributed to their narrow therapeutic index (TI).
- Under such circumstances, the TI can be improved by minimizing the delivery of drug to normal cells by encapsulating in liposomes.
- Free doxorubicin has a severe side effect of cardiac toxicity, but when formulated as liposomes, the toxicity was reduced without any change in the therapeutic activity
Site specific targeting
- Delivery of larger fraction of drug to the desired (diseased) site, by reducing the drug’s exposure to normal tissues can be achieved by site specific targeting.
- Encapsulating the drug in liposomes can be used for both active and passive targeting of drugs in order to achieve a safer and efficacious therapy
- On systemic administration, long circulating immunoliposomes are able to recognize and bind to target cells with greater specificity.
Intracellular drug delivery
- Increased delivery of potent drugs to the cytosol (in which drug’s receptors are present), can be accomplished using liposomal drug delivery system.
- N-(phosphonacetyl)-L-aspartate (PALA) is normally poorly taken up into cells. Such drugs when encapsulated within liposomes, showed greater activity against ovarian tumor cell lines in comparison to free drug
Sustained release drug delivery
- Liposomes can be used to provide a sustained release of drugs, which require a prolonged plasma concentration at therapeutic levels to achieve the optimum therapeutic efficacy
- Drugs like cytosine Arabinoside can be encapsulated in liposomes for sustained release and optimized drug release rate in vivo
IntraperitoneaI administration
- Tumors that develop in the intra-peritoneal (i.p.) cavity can be treated by administering the drug to i.p. cavity.
- liposomal encapsulated drugs have lower clearance rate, when compared to free drug and can provide maximum fraction of drug in a prolonged manner to the target site
Immunological adjuvants in vaccines
- Immune response can be enhanced by delivering antigens encapsulated within liposomes.
- Depending on the lipophilicity of antigens, the liposome can accommodate antigens in the aqueous cavity or incorporate within the bilayers
- In order to enhance the immune response to diphtheria toxoid, liposomes were first used as immunological adjuvants.
