Metronomic Chemotherapy
Cancer
- Every year globally 8.2 million people die from cancer
- ICMR in 2016 said that the total number of new cancer cases in India is likely to reach nearly 17.3 lakh by 2020
- According to a study, 7.5% of patient deaths occurred within 30 days of chemotherapy, were related to treatment rather than disease progression
Current challenges to Maximum Tolerated Dose (MTD) Chemotherapy
(maximum tolerated dose is determined in clinical trials by testing increasing doses on different groups of people until the highest dose with acceptable side effects is found)
- Conventional approach entails administering the drugs at doses close to the maximum tolerated dose (MTD). à side effects
- Also, it consists of time interval ranging between two and four weeks to allow for the recovery of the normal tissues, mainly the bone marrow progenitors. S/E on bone marrow progenitors, gut mucosal cells or hair follicle cells (DNA damage / microtubule inhibition)
- Also, this strategy potentially allows regrowth of the tumor in the interval period and leads to the emergence of resistant populations of tumor cells.
- Targeting endothelial cells present in a tumor’s growing vasculature à Potential Benefit (Tumor thrives on vascular supply, hence why not target that?)
What would be the advantage of using chemotherapeutics as possible angiogenesis inhibitors?
- High CT doses required an extended treatment-free period to permit recovery of normal host cells, e.g., rapidly growing hematopoietic progenitors. Similar to hematopoietic progenitors, the vascular endothelial cells in the tumor bed might also resume growth during this treatment-free period.
- Hypothesis à endothelial cell recovery occurring during this treatment-free period could support regrowth of tumor cells. This could increase the risk of the emergence of drug-resistant tumor cells.
- Researchers à established that angiogenesis is the key factor in the local and metastatic growth of cancer.
- The discovery of the pivotal role of angiogenesis in tumor growth. The scientific basis for metronomic chemotherapy is that conventional anti‑neoplastic drugs target vascular endothelial cell proliferation but the anti‑angiogenetic effect cannot be sustained because endothelial cells get a chance to recover during treatment breaks and this may be overcome by frequent treatment at low doses.
Why not routine angiogenesis inhibitors?
Shortcomings of anti-vascular tumor therapy:
(i) most tumors are inherently resistant to VEGFi and other anti-vascular therapies used alone;
(ii) even when used in combinations that increase the initial response rate, responsive tumors typically develop acquired resistance within a few months; and
(iii) Adjuvant use of these agents did not increase cure rates.
However, the frequent and sustained use of low doses of conventional chemotherapeutics mimics the long-term antiangiogenic activities of VEGFi.
- anti-angiogenic effects of conventional anti-angiogenic drugs, which target individual molecules or signaling pathways
- anti-angiogenic actions of metronomic chemotherapy, which inhibit the production of growth factors at the source.
- For instance, bevacizumab, an anti-angiogenic monoclonal antibody, binds to extracellular VEGF, rendering it incapable of activating cell surface VEGF receptors and thus incapable of initiating sprout formation.
- In contrast, metronomic chemotherapy damages the source of these growth factors, namely, fibroblasts and TECs.
- Therefore, while metronomic chemotherapy and anti-angiogenic drugs can both induce anti-angiogenesis, the underlying mechanisms are different, with metronomic therapy potentially having more lasting effects due to its targeting the source of vascular growth factors rather than the growth factors themselves.
Klement et al., demonstrated that continuous low‑dose vinblastine combined with anti‑VEGF antibody (VEGF: Vascular endothelial growth factor) caused a significantly greater regression of xenograft tumors as a result of reduced tumor vascularity and angiogenesis.
Concept of Metronomic Chemotherapy
- Cyclophosphamide administered chronically once a week without breaks
- At a lower dose (e.g. one third of the MTD)
- Repair process was compromised and anti-angiogenic effects of the drug were not lost.
- This method of administrating chemotherapy was named ‘anti-angiogenic chemotherapy’ by Browder et al and ‘metronomic’ dosing by Hanahan et al
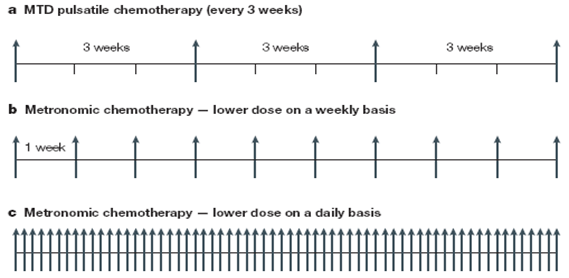
Metronomic chemotherapy is the chronic administration of chemotherapy at low, minimally toxic doses on a frequent schedule of administration, with no prolonged drug-free breaks.
- Frequent (dose-dense) administration of chemotherapy without any interruptions
- Using a biological optimized dose instead MTD
- Preference for oral drugs
- Low incidence of treatment related side-effects
- Potential for delayed development of resistance.
- Have been tested both as single agent and combination chemotherapy
- The word Metronomic has been derived from ‘Metronome’ = musical instrument that produces regular, metrical ticks representing fixed, regular aural pulse.
Mechanism of action
Metronomic chemotherapy induces important antiangiogenic effects (inhibition of endothelial cell proliferation, migration and morphogenesis, decrease in mobilization and viability of endothelial progenitor cells and increase in Thrombospondin-1 expression), resulting in a reduction of the tumor vasculature.
In addition, metronomic chemotherapy also decreases the number and activity of regulatory T cells (TREG) and may promote dendritic cell maturation, leading to (re)activation of an anticancer immune response, in part mediated by cytotoxic T cells and natural killer (NK) cells.
4 D Effect
Andre et al., have postulated a ‘drug‑driven dependency/ deprivation’ or a 4‑dimensional (4D) phenomenon
- Tumor cells become dependent on the chemotherapy during long exposure
- Sudden cessation or replacement of therapy might lead to cell death.
| Conventional Chemotherapy | Metronomic Chemotherapy |
| Maximum tolerated doses(MTD) used | Lower dose than MTD |
| Therapy at defined intervals depending on recovery of bone marrow. Eg: 3 weekly | Dosing frequency is continuous. Eg: Weekly, daily, alternate days |
| Rise and fall of plasma conc | Sustained plasma conc |
| Targets proliferating tumor cells | Targets endothelial cells of vasculature of the tumour |
| Toxicity concern | Less toxicity |
Criteria of an anti-angiogenic agent for MCT
- Strong differential cytotoxicity between cancer cells and endothelial cells
- Changes of mechanistic effects (e.g., biomarker changes:IL‑1 and 6, VEGF, VEGFR1 and 2, bFGF, MMP‑2 and 9, vessel density etc.)
- Inhibition of angiogenesis in‑vivo and in‑vitro (in‑vivo models at best only with spontaneous, slow growing tumors)
Which Patients Are Candidates?
- Does not benefit every patient as is clear from the clinical data gathered to date.
- Need to identify the right context and the right patient group to benefit from metronomic chemotherapy
- whether in mono- or combination therapy.
When to use?
Palliative purposes in relapse/refractory diseases and metastatic cases
- Particularly appropriate for maintenance strategies
- Can be delivered as a continuation of the induction regimen, where one or two drugs already used in the induction regimen are carried on as maintenance;
or
- As switch maintenance, where short periods of conventional chemotherapy are followed by long courses of non-cross-resistant cytotoxic drugs.
Toxicity
- Generally, well tolerated.
- Most common toxic effects of this treatment are:
- Grade 1 nausea and/or vomiting,
- Grade 1 and 2 anemia, neutropenia, leucopenia and lymphopenia as well as low-grade fatigue
- Cumulative effects can lead to secondary leukemia, or myelodysplastic syndrome (MDS)
Biomarkers for evaluation
Biomarkers to indicate achievement of pharmacodynamic effects are important to determine the optimal metronomic dose (OMD) of cytotoxics.
MTD determination is easily established with routine laboratory tests and clinical assessments, OMD determination faces significant challenges
the circulating endothelial progenitor cells (ceps) that are considered to be an alternative source for some of the endothelial cells of newly formed blood vessels represent a subset of immature vegfr2-positive cecs also found in peripheral blood

Biomarkers
- Circulating blood biomarkers (cytokines such as VEGF, thrombospondin-1/2 and circulating endothelial cells)
- Functional imaging (e.g. DCE-MRI, or DCE-CT – utilized in early phase clinical trials)
- However, these biomarkers have not shown to consistently correlate with response or survival outcome.
- Quantitative parameters of tumor vascularity derived using DCE-MRI include blood flow, permeability-surface area product, fractional intravascular volume and fractional interstitial volume. However functional imaging is limited by the fact that only one or two representative lesions from one tumor site can be selected for measurement and it is needful therefore to assume that changes in levels in the selected lesion reflect similar changes in other unmeasured lesions during antiangiogenic treatment. Therefore, functional imaging fails to take into account the possible heterogeneity in behavior of the disseminated lesions.
Trials in Metronomic CT
- Metronomic doses are nearly 1/10th of MTD of conventional chemotherapy – Toxicity not a concern
- Therefore, the aims of phase 1 clinical trial is to obtain the Optimum Biological Dose (OBD) of a drug
- Phase I standard ‘3+3’ design to observe pre-defined DLT à Failed to detect the OBD.
- The OBD is determined based on the performance of desired level of Surrogate Marker (SM).
Single arm studies
- Majority of these are Phase I and Phase II studies, with small patient numbers
- often when standard of care has been exhausted.
- Data from Indian studies (n = 30) include 1390 patients, with head and neck cancer patients (n = 544) and breast cancer (n = 260) being the most common.
- The most common metronomic therapy combination used methotrexate and celecoxib, drugs that are easily available and inexpensive. Other most commonly employed drugs were cyclophosphamide, methotrexate, capecitabine, bevacizumab, vinorelbine
- Colon cancer, prostate cancer and ovarian cancers using metronomic regimen showed favourable outcome in their endpoints.
Issues in metronomic trials
- Lack of studies regarding pharmacokinetics and the pharmacodynamic properties
- To detect cumulative side effects, Long duration of trials– How long?
- Metronomic chemotherapy studies should be adopted only after strong preclinical data indicating –
- Which drugs should be used, how long, and at which doses?
- Role of pharmacologist- Need for PK/PD studies – relevance of such knowledge in designing clinical trials to move away from empirical research primarily due to an underestimation of the importance of plasma/tumour concentrations of drugs and their biological effects.
Metronomic Resistance
Contrary to initial expectations, antivascular therapies are equally prone to inherent or acquired resistance as other cancer treatment modalities.
Typically acquired resistance ensues within months
Cost Comparison
- According to a pharmacoeconomic evaluation by Bocci et al. in metastatic breast cancer, metronomic regimen is a cost-effective alternative to intravenous infusion chemotherapy regimens
- Oral administration, decreased hospitalization etc
- Do not need to travel as can be taken at home
- Low toxicity so no further added costs to manage toxicities such as infection, renal toxicities
Drug repositioning
- Using drugs already approved for non-malignant diseases on the basis of newly identified anticancer properties
- These drugs have side-effects that are known, usually moderate, and well documented.
- Phase 1 studies are therefore not mandatory and further clinical development can often start directly with phase 2 trials
- ReDo Project- Repurposing Drugs in Oncology (ReDO) project
- To address the issues around the re-use of non-cancer drugs in oncological treatments
Examples – Celecoxibà Anti-angiogenic
Propranolol à Immunomodulatory and anti-angiogenic properties
Valproic acid à Histone deacetylase inhibitor
Metformin à AMP kinase and mTOR inhibitor or epithelial–mesenchymal transition inhibitor
Limitations
- Most effective dose and schedule have yet to be defined
- Metronomic chemotherapy would not benefit every patient as is clear from the clinical data gathered to date.
- Need to identify the right context and the right patient group to benefit from metronomic chemotherapy whether in mono- or combination therapy.
- Time lag between anti-tumor effect and a visible reduction in tumor bulk may in some cases decrease the utility of metronomic chemotherapy for advanced disease.
