Nano-pharmacology
Nanotechnology is continuously being explored in the field of medicine with the objective of maximizing current therapeutic strategies and minimizing side effects of drugs. Nano-pharmacology is concerned with the application of nanotechnology on the basis of novel pharmacological principles with a view to increase therapeutic efficacy and reduce side effects, and to achieve targeted delivery of medicine to specific sites in a controlled manner.(1)
Integration of nanotechnology with pharmacology for enhanced chemotherapy in multidrug-resistant cancer:Multidrug resistance (MDR) in cancer is a common clinical problem. It involves complex cellular and non-cellular mechanisms (e.g. the aberrant tumour micro-environment i.e. TME), which can collectively contribute to reduced drug concentrations, activities and efficacy at tumour sites and within cancer cells, leading to ineffective chemotherapy.(2) To overcome the multifactorial MDR mechanisms for improved chemotherapy, various approaches have been investigated, such as the development of new anticancer drugs that are poor substrates for efflux transporters, downregulation of MDR-associated enzymes using RNA interference; synergistic drug combination with different mechanisms of drug actions and multifunctional nanoparticles (NPs). Solid polymer-lipid hybrid NPs (PLNs) have been designed to bypass efflux transporters and synergistic drug combinations or drug-nanomaterial combinations in NPs have been explored.(3) Once administered intravenously into the body, the drug combination non-specifically distributes to normal organs before reaching the tumour due to the different pharmacokinetic profiles of each drug, resulting in increased adverse effects. Application of nanotechnology in drug delivery makes it possible to deliver synergistic drug combinations ratiometrically and spatial-temporally to the sites of drug action(4). The use of nanosized drug carriers, several nanoparticle formulations of synergistic drug combination have been moved to clinical trials(5) or have gained approval by the United States Food and Drug Administration (US-FDA) or other regulatory agencies(6).
Harnessing intracellular drug-drug interactions to design synergistic nanomedicine: It is known that under the hypoxic conditions of the TME, the cytotoxicity of Doxorubicin (DOX) is attenuated, as its activity partially relies on the presence of oxygen to generate free radicals that can cause DNA damage. In contrast, mitomycin-c (MMC) can be activated under hypoxia through a sequential one electron reduction pathway that enhances its cytotoxicity and can generate free radicals in the presence of oxygen(7). In addition, DOX and MMC damage DNA by different mechanisms: DOX causes DNA double-strand breaks by poisoning topoisomerase IIα an enzyme responsible for catalyzing the unwinding of DNA for transcription and replication, whereas MMC induces DNA intra-strand and inter-strand crosslinks to disrupt cell maintenance and replication(8). However, the clinical combination of DOX and MMC (DOX-MMC) was halted owing to its severe cardiotoxicity to cancer patients. This off-target adverse effect could be reduced with optimal scheduling and dosing of the two drugs by rationally designing a drug nanocarrier system(9). PLN formulation coloaded with DOX- MMC (DMPLN) designed with a key capability of synchronizing co-delivery of locally bioavailable DOX-MMC combination at synergistic ratios to cancer cells. As a result, DMPLN provided superior advantages over free DOX-MMC solution and liposomal DOX with enhanced tumour cell apoptosis and minimum cardiotoxicity, as well as prolonged host-survival in both human and murine MDR tumour models(10).
Harnessing naturally occurring lipoprotein to design self-decorating nanoparticles for drug delivery to the brain: Nearly 15%–30% of patients with advanced breast cancer develop brain metastases during progression of the disease. Brain metastases are associated not only with a poor prognosis but also with severe neurological impairment. The treatment of brain metastases is challenging due to the inability of most therapeutic agents to penetrate the BBB, leading to subtherapeutic drug accumulation in the CNS. The drug efflux transporters found on brain endothelial cell membranes of BBB further expel substrate molecules, including many effective anticancer drugs from the brain. As a result, it restricts passive entry of nearly 98% of all drugs to the brain at the blood-brain interface(11). Therefore, development of effective strategies for shuttling drugs across the BBB has been a long-standing goal in the drug delivery field. Of the various receptor-mediated brain-penetrating systems, NPs present more advantages than antibody-drug conjugates. Nanoparticle carriers can be engineered by surface decoration of targeting antibodies or ligands via covalent bonds or physical coating. Compared to pre-engineered nanocarrier systems, NPs that are able to adsorb receptor-binding moieties from the blood during circulation are more attractive owing to their low immunogenicity and cost effectiveness. Delivery of a therapeutic antibody to the brain requires particular considerations differing from the delivery of small molecules. To deliver therapeutic antibody TRA to human epidermal growth factor receptor-2 (HER2)-positive cancer cells in the brain metastasis area, an intracellularly eroded solid-lipid polymer matrix containing a TRA-conjugated terpolymer can be used. The nanoconstructs were able to shield TRA in the circulatory system and deliver it to brain tumours via a two-step targeting process. The constructs remained intact during systemic circulation, crossed the BBB (i.e. the first step in targeting the brain), and then n dissociated and exposed TRA, enabling the second-step targeting of TRA to the cancer cells within the brain and thus generating a therapeutic effect(12).
Integration of nanotechnology with pathophysiological features for disease-responsive drug delivery and enhanced radiation therapy: Various nanotechnology-based strategies have been developed to modulate TME, including 1) Nanoparticle formulations of angiogenesis inhibitors or small interfering RNA (siRNA) against vascular endothelial growth factor receptor-2 (VEGFR-2) to remodel the tumour vasculature, 2) Compounds for reducing/degrading collagen content in the tumour extracellular matrix, and 3) Cytokines to modulate the phenotype of tumour-associated macrophages (TAMs)(13). Preclinical studies using orthotopic breast tumour models demonstrated good tumour accumulation and sustained effects of the lipid-polymer-based MDNPS (LMD NPs) on modulating TME factors(14).
Mimicking dynamic insulin release from human pancreatic cells: In healthy individuals, insulin secretion by pancreatic β-cells is synchronized with glucose level via a quick pulsatile surge when the glucose level rises after a meal and brings it down to euglycemia within 30–60 min.(15) In type 1 diabetes patients mimicking this dynamic insulin release pattern is critical to avoid extended tissue exposure to hyperglycemia and the risk of hypoglycemia. However, even with frequent self-monitoring of glucose level and administration of anticipated amounts of insulin, conventional insulin therapy is far from the ideal physiological response. Therefore, closed-loop insulin delivery systems have been developed to automate these steps with the use of continuous blood glucose monitors and insulin pumps to better emulate physiological conditions and minimize user’s intervention. However, issues regarding the cost of the pumps and accessories and the short usage duration (3–7 d) of the accessories remain to be resolved(16). Recent advances in nanomaterials offer new opportunities for developing next-generation closed-loop insulin delivery systems that better mimic the endocrinological secretion of insulin. Our laboratory introduced pH-responsive hydrogel NPs to replicate the rapid and dynamic insulin release at physiologically relevant time scales. After implantation of hydrogel NPs in T1D rats, this nanotechnology-enabled insulin delivery system was able to reproducibly bring elevated blood glucose levels down to normal levels within 30 min after induced hyperglycemia, which mimics the endogenous insulin response time of healthy rats and resembles the pattern in healthy humans(17).
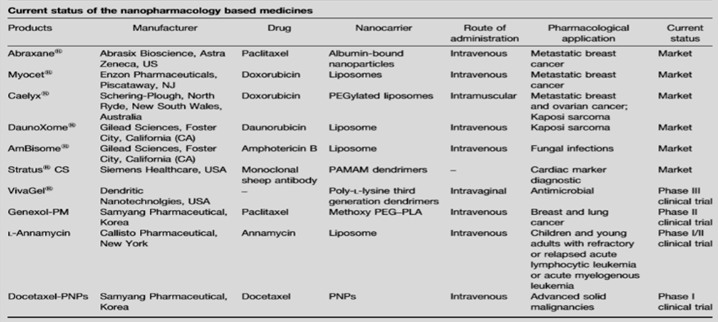
Current status of the nanopharmacology based medicines: (18)
References:
1) Florence AT: ‘‘Targeting’’ nanoparticles: the constraints of physical laws and physical barriers. J Control Release 2012, 164:115-124.
2) Wong HL, Bendayan R, Rauth AM, Li Y, Wu XY. Chemotherapy with anticancer drugs encapsulated in solid lipid nanoparticles. Adv Drug Deliv Rev 2007; 59: 491–504.
3) Zhang RX, Cai P, Zhang T, Chen K, Li J, Cheng J, et al. Polymerlipid hybrid nanoparticles synchronize pharmacokinetics of co-encapsulated doxorubicin-mitomycin C and enable their spatiotemporal co-delivery and local bioavailability in breast tumor. Nanomedicine 2016; 12: 1279–90.
4) Zhang RX, Wong HL, Xue HY, Eoh JY, Wu XY. Nanomedicine of synergistic drug combinations for cancer therapy – strategies and perspectives. J Control Release 2016; 240: 489–503.
5) Batist G, Gelmon KA, Chi KN, Miller WH Jr, Chia SK, Mayer LD, et al. Safety, pharmacokinetics, and efficacy of cpx-1 liposome injection in patients with advanced solid tumors. Clin Cancer Res 2009; 15: 692–700.
6) US Food & Drug Administration. Updated 2017 Aug 4; cited 2018 Jan 08. FDA approves first treatment for certain types of poorprognosis acute myeloid leukemia.
7) Rauth AM, Melo T, Misra V. Bioreductive therapies: An overview of drugs and their mechanisms of action. Int J Radiat Oncol Biol Phys 1998; 42: 755–62.
8) Wang P, Song Y, Zhang L, He H, Zhou X. Quinone methide derivatives: Important intermediates to DNA alkylating and DNA cross-linking actions. Curr Med Chem 2005; 12: 2893–913.
9) Shuhendler AJ, Cheung RY, Manias J, Connor A, Rauth AM, Wu XY. A novel doxorubicin-mitomycin C co-encapsulated nanoparticle formulation exhibits anti-cancer synergy in multidrug resistant human breast cancer cells. Breast Cancer Res Treat 2010; 119: 255–69.
10) Shuhendler AJ, Prasad P, Zhang RX, Amini MA, Sun M, Liu PP, et al. Synergistic nanoparticulate drug combination overcomes multidrug resistance, increases efficacy, and reduces cardiotoxicity in a nonimmunocompromised breast tumor model. Mol Pharm 2014; 11: 2659–74.
11) Saraiva C, Praca C, Ferreira R, Santos T, Ferreira L, Bernardino L. Nanoparticle-mediated brain drug delivery: Overcoming blood-brain barrier to treat neurodegenerative diseases. J Control Release 2016; 235: 34–47.
12) He C, Li J, Cai P, Ahmed T, Henderson JT, Foltz WD, et al. Two-step targeted hybrid nanoconstructs increase brain penetraion and efficacy of the therapeutic anitbody trastuzumab against brain metastasis of Her2 positive breast cancer. Adv Funct Mater 2018; 28: 1705668.
13) Yang S, Gao H. Nanoparticles for modulating tumor microenvironment to improve drug delivery and tumor therapy. Pharmacol Res 2017; 126: 97–108.
14) Abbasi AZ, Gordijo CR, Amini MA, Maeda A, Rauth AM, DaCosta RS, et al. Hybrid manganese dioxide nanoparticles potentiate radiation therapy by modulating tumor hypoxia. Cancer Res 2016; 76: 6643– 56.
15) DiPiro JT, Talbert RL, Yee GC, Matzke GR, Wells BG, Posey LM, editors. Pharmacotherapy: A pathophysiologic approach. 8th edition. McGraw-Hill Education; 2011.
16) Pozzilli P, Battelino T, Danne T, Hovorka R, Jarosz-Chobot P, Renard E. Continuous subcutaneous insulin infusion in diabetes: Patient populations, safety, efficacy, and pharmacoeconomics. Diabetes Metab Res Rev 2016; 32: 21–39.
17) Chu MK, Gordijo CR, Li J, Abbasi AZ, Giacca A, Plettenburg O, et al. In vivo performance and biocompatibility of a subcutaneous implant for real-time glucose-responsive insulin delivery. Diabetes Technol Ther 2015; 17: 255–67.
18) Jain K, Mehra NK, Jain NK. ScienceDirect Potentials and emerging trends in nanopharmacology. Curr Opin Pharmacol [Internet]. 15:97–106.
