Pharmacotherapy of Obesity
Introduction
- Overweight and obesity: defined as abnormal or excessive fat accumulation resulting from chronic imbalance of energy whereby the intake of energy exceeds expenditure.
Multifactorial disorder
- Genetic
- Dietary
- Lifestyle
- Deficiency in synthesis/action of hormones
- Hypothalamic neuronal system defect
- Drug induced
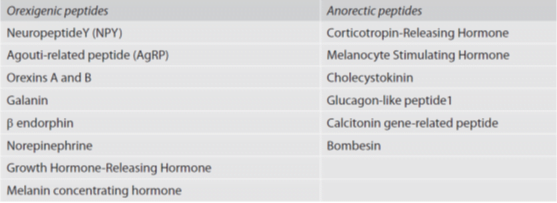
• Leptin is synthesized and secreted primarily from adipocytes and acts centrally in the hypothalamus by binding to the leptin receptor. Circulating levels of leptin are highly correlated with the level of body fat.
• Insulin and glucocorticoids can stimulate production of leptin by adipocytes. Low plasma concentrations of leptin and insulin, e.g. during fasting and weight loss, increase food intake and decrease energy expenditure by stimulating neuropeptide Y (NPY) synthesis, and perhaps by inhibiting sympathetic activity and other catabolic pathways.
• High leptin and insulin concentrations, e.g. during feeding and weight gain, decrease food intake and increase energy expenditure through release of melanocortin and corticotropin-releasing hormone (CRH).
• Stimulation of the leptin receptor can lead to changes in the expression of a variety of neuropeptides.
• Neuropeptides that are involved in energy homeostasis can be orexigenic (appetite stimulating) and anorectic
WHO- “Epidemic of 21st century
- WHO Asia Pacific guidelines:
- overweight (BMI ≥ 23 kg/m2 but <25 kg/m2),
- generalized obesity (GO, BMI ≥ 25kg/m2),
- abdominal obesity (AO, waist circumference ≥90 cm for men and ≥ 80cm for women)
- combined obesity (CO, GO plus AO).
Goal of therapy
The goal of any treatment (including drug therapy) for overweight individuals is to reduce weight and improve overall health
●Reduce weight –
- In short-term (6 to 12 months) à drug therapy, weight loss of 4 to 8 percent is typical
- Drug therapy does not cure obesity.
- Patients with obesity given drugs should be advised that when the maximal therapeutic effect is achieved, weight loss ceases. When drug therapy is discontinued, weight is expected to rise.
- Achieving and maintaining weight loss
●Improve health status –
- improvement in physical function, comorbidities, and/or sense of well-being.
- Weight loss should exceed 2 kg during the first month of drug therapy (1 pound per week), fall more than 4 to 5 percent below baseline between three to six months, and remain at this level to be considered effective.
●Minimize adverse effects –
- The potential benefits of weight loss must be considered in light of the potential risks of drug therapy.
- The longest clinical trial examining the safety and efficacy of pharmacotherapy for weight loss utilized orlistat for four years. Thus, in patients wishing to use anti-obesity medication for longer than four years, the lack of longer-term safety (and efficacy) data should be made known.
General principles
●Initial management –
- comprehensive lifestyle intervention: a combination of diet, exercise, and behavior modification.
- Earnest counseling around healthy eating, physical activity, and health-seeking behavior is essential for every patient seeking weight loss, whether used alone or in combination with weight-loss medication or bariatric surgery.
●Approach to underlying comorbidities –
- An important component of the initial evaluation àthe assessment of obesity-related comorbid conditions such as diabetes mellitus, dyslipidemia, hypertension, heart disease, sleep apnea, and symptomatic osteoarthritis.
- Several drugs are well known to produce weight gain and should be avoided if good alternatives are available Medications used to treat diabetes, depression, and autoimmune diseases are particularly notorious for causing weight gain.
Candidates for drug therapy —
- individuals with a body mass index (BMI) ≥30 kg/m2, or
- a BMI of 27 to 29.9 kg/m2 with comorbidities, who have not met weight-loss goals (loss of at least 5 percent of total body weight at three to six months) with a comprehensive lifestyle intervention.
- The decision to initiate drug therapy à individualized and
- careful evaluation of the risks and benefits of all treatment options (lifestyle, pharmacologic, surgical).
Choice of agent —
Pharmacologic options
- lorcaserin,
- liraglutide (daily injection),
- orlistat,
- combination phentermine-extended release topiramate (in one capsule),
- combination bupropion-naltrexone (in one extended-release tablet),
- phentermine,
- benzphetamine,
- phendimetrazine, and
- diethylpropion
Single agents are preferred over combination medications as initial pharmacotherapy.
- Serotonon agonist – receptor 2C
- has similar efficacy to orlistat, with a more favorable side effect profile.
- beneficial effects on glycemia and kidney function and may be used in patients (with or without diabetes) who have cardiovascular disease or risk factors for cardiovascular disease, including hypertension and dyslipidemia.
Pharmacology —
Serotonin reduces food intake in animals and human beings; thus, agonists to appropriate serotonin receptors are potentially valuable drugs.
It reduces appetite and thereby reduces body weight in men and women
Nonselective serotonergic agonists, such as fenfluramine and dexfenfluramine, also enhanced weight loss in clinical trials. However, they increased the risk of serotonin-associated cardiac valvular disease, thought to occur through activation of serotonin receptor 2B.
Metabolic effects —
Lorcaserin has beneficial effects on several metabolic parameters, including glucose control, kidney function, and possibly blood pressure and low-density lipoprotein (LDL) cholesterol.
Adverse effects —
generally mild and included headache, upper respiratory infections, nasopharyngitis, dizziness, and nausea,
Dosing and contraindications —
recommended dose à 10 mg twice daily, taken with or without food, and there is no need for a titration period.
Lorcaserin should not be used in individuals with CrCl <30 mL/min.
It is contraindicated during pregnancy.
lorcaserin should not be used with other serotonergic drugs (eg, SSRI, SNRI, bupropion, TCA, and MAOI), because of the theoretical potential for serotonin syndrome
- glucagon-like peptide-1 [GLP-1] agonist
- stimulate glucose-dependent insulin secretion.
- has beneficial effects on glycemia,
- GLP-1 also inhibits glucagon release and gastric emptying
- They are used in combination with metformin (and/or another oral agent) for patients with type 2 diabetes who fail initial therapy with one or two oral agents, particularly when weight loss is a primary consideration
- prefer to use it in obese patients with type 2 diabetes where its side effects, need for injections, and expense are balanced by improved glycemia and weight loss.
- Cardiovascular effects — Although liraglutide has been shown to reduce major cardiovascular disease events in one trial, those results were generated in a type 2 diabetes population where obesity was not an entry requirement, and the dose of liraglutide used was lower than the dose recommended for weight loss (1.8 versus 3 mg)
- Therefore, cardiovascular disease reduction cannot be used as a rationale for using liraglutide primarily for weight loss.
- However, gastrointestinal side effects (nausea, vomiting), the need for a daily injection, and insurance coverage/cost may limit the use of this drug
Adverse events —
- Gastrointestinal side effects, including nausea and vomiting, are common.
- weight loss may be due, in part, to gastrointestinal side effects directly or through suppression of appetite.
- Other side effects include diarrhea, low blood sugar, and anorexia.
Dosing and contraindications —
- Liraglutide à subcutaneously in the abdomen, thigh, or upper arm once daily.
- initial dose à 0.6 mg daily for one week.
- increased at weekly intervals (1.2, 1.8, 2.4 mg) to the recommended dose of 3 mg
- contraindicated during pregnancy and in patients with a personal or family history of medullary thyroid cancer or multiple endocrine neoplasia 2A or 2B.
- alters fat digestion by inhibiting pancreatic lipases
- fecal fat excretion is increased
- proven benefits with regard to glycemia, lipids, and blood pressure.
- There are long-duration trials with orlistat demonstrating its safety profile.
- Cardiovascular effects — In hypertensive patients, orlistat improves blood pressure (likely due to weight loss),
- In addition, orlistat improves some serum lipid values more than can be explained by weight reduction alone
Dosing— 120 mg three times daily.
- Unfortunately, it frequently causes gastrointestinal side effects and is often not tolerated by patients.
- s/e – intestinal borborygmi and cramps, flatus, fecal incontinence, oily spotting, and flatus with discharge
- Severe liver injury has been reported rarely with the use of orlistat
- ●Absorption of fat-soluble vitamins – In a meta-analysis, levels of fat-soluble vitamins (A, D, E, K) and beta-carotene were lowered by orlistat therapy, with vitamin D the most frequently affected.
- for patients taking warfarin, a decrease in vitamin K may necessitate a reduction in the dose of warfarin
- ●Renal – Oxalate-induced acute kidney injury has also been reported in orlistat users. Malabsorption syndromes are a risk factor for calcium oxalate stones
- patients who take orlistat should contact their health care provider if itching, jaundice, pale color stools, or anorexia develop.
- Due to its limited tolerability, and the established safety and benefits of other available agents including liraglutide and lorcaserin, we no longer consider orlistat to be first-line pharmacotherapy.
contraindications —
should not be used during pregnancy or in patients with chronic malabsorption, cholestasis, or a history of calcium oxalate stones.
●Combination phentermine-topiramate (extended release)
- option for men or postmenopausal women with obesity WITHOUT hypertension or coronary heart disease, particularly those who do not tolerate orlistat, lorcaserin, or liraglutide.
- The efficacy for weight loss à greater than for either orlistat or lorcaserin,
- The efficacy and safety of combining generic phentermine with generic topiramate for weight loss (each taken individually) has not yet been established.
- but it may have more side effects (eg, increased heart rate, dose-related increase in the incidence of psychiatric [eg, depression, anxiety] and cognitive [eg, disturbance in attention] adverse events).
- may be an acceptable option for a patient with an obesity-related comorbidity, such as sleep apnea, who does not have any cardiovascular disease.
Adverse effects —
dry mouth, constipation, and paresthesia
dose-related increase in the incidence of psychiatric (eg, depression, anxiety) and cognitive (eg, disturbance in attention)
Dosing and contraindications —
initial dose of à 3.75/23 mg for 14 days, followed by 7.5/46 mg thereafter.
If an individual does not lose 5 percent of body weight after 12 weeks on the highest dose, phentermine-topiramate should be discontinued gradually as abrupt withdrawal of topiramate can cause seizures
- The presence of topiramate in this combination may increase risk of fetal malformations, and it should thus be used with caution in women of childbearing age, who should have a pregnancy test before its use and monthly thereafter.
●Combination bupropion-naltrexone (sustained release)
- produces similar weight loss as orlistat and lorcaserin,
- but it has more side effects and contraindications
- not first line
- could be prescribed for the obese smoker who desires pharmacologic therapy for smoking cessation and obesity.
- Owing to the uncertainty about cardiovascular effects, we prefer to use orlistat, liraglutide, or lorcaserin, rather than bupropion-naltrexone.
- Because bupropion-naltrexone can raise blood pressure and heart rate, the FDA is requiring post-marketing studies to evaluate cardiovascular outcomes and the effect of the combination on cardiac conduction
Dosing and contraindications —
initial dose is one tablet (8 mg of naltrexone and 90 mg of bupropion) daily.
After one week, the dose is increased to one tablet twice daily and, by week four, to two tablets twice daily.
Contraindications include pregnancy, uncontrolled hypertension, seizure disorder, eating disorder, use of other bupropion-containing products, chronic opioid use, and use within 14 days of taking monamine oxidase inhibitors
●Phentermine, benzphetamine, phendimetrazine, and diethylpropion are only approved by the US Food and Drug Administration (FDA) for short-term use and have more side effects, and there is potential for abuse
Sympathomimetic drugs reduce food intake by causing early satiety.
- some clinicians and their patients choose to use phentermine for longer periods of time, owing to long-term clinical experience with this drug.
Monitoring
●Weight, vital signs –
- After initiating pharmacologic therapy, we monitor weight loss, blood pressure, and heart rate on a weekly basis for four weeks and then monthly for the next three to four months, at which time a decision should be made whether to continue the drug.
- If patients do not lose 4 to 5 percent of body weight after 12 weeks of tolerated maximum-dose therapy, the drug should be tapered and discontinued.
- Although it is uncertain whether people who fail to respond to one drug will respond to another (or to a combination drug
●Blood sugar in patients with diabetes –
- Weight loss may cause hypoglycemia in patients taking medication for diabetes, especially insulin or insulin secretagogues (eg, sulfonylureas, meglitinides), and in such patients, self-monitoring of blood glucose (SMBG) should be performed more frequently for safety.
●Adverse effects –
- ask about adverse effects during every visit.
- lorcaserin, phentermine-topiramate, and bupropion-naltrexone à neuropsychiatric side effectsà monitored for depression or suicidal thoughts.
- liraglutide à monitored for symptoms of acute pancreatitis and gallbladder disease.
- Hyperchloremic, non-anion gap metabolic acidosis and increases in serum creatinine have been reported in patients treated with phentermine-topiramate because topiramate is a carbonic anhydrase inhibitor. Thus, serum electrolytes (including bicarbonate) and creatinine should be measured before and approximately four weeks after initiation of this combination.
Recent
- Melanocortin 4 receptor (MC4R)- Setmelanotide:
- MC4R mutations: most common monogenetic cause of obesity in humans
- RM-493: selective MC4R agonist, subcutaneous, increased resting energy expenditure in randomized, placebo-controlled study [
- Peptide of MC4R: promising results in rodents, soon to enter phase 1 trial
- Setmelanotide: Patients with POMC defects à significantly more weight loss than MC4R deficient patients or obese controls (phase 1b)
- NPY receptor ligands – antagonist
- Neuropeptide (NPY): stimulate appetite by activation of Y1 and Y5 receptors
- Antagonist: aim of blocking orexigenic effect
- Molecules under Phase I and Phase II
- Glucagon like peptide 1 receptor agonist
- Exenatide: Approved for treatment of DM2, not licensed to treat obesity
- Tapsoglutide: promising in pre clinical study; dropped in phase 3 due to diverse GI events and hypersensitivity issues
- Oxyntomodulin: iv infusion reduces food intake; repeated sc injections increases energy expenditure and causes weight loss
- Pegylated form: PF- 05212389 also in phase 1 trial
- Fatty acid synthase (FAS): catalyses de novo synthesis of saturated fatty acids, such as plamitate and stearate
Inhibitors: cerulenin and C75
- Acetyl-CoA carboxylase (ACC): ACC1 isoform regulates fat synthesis
Inhibitor: CP-640186
- β3 receptor agonist:
- Enhancement of lipolysis in white fat
- Thermogenesis in skeletal muscle/brown fat
- Agonists: Mirabegron (marketed – overactive bladder), Solabegron (Phase II – Overactive bladder, IBS)
- Amibegron, BTA243 (discontinued – Obesity)
- Amylin analogs
- Pramlintide: synthetic analogue of amylin for diabetic patients as an adjunct therapy to mealtime insulin
- Phase IIA study with overweight and obese subjects treated with pramlintide & recombinant leptin: pramlintide restores sensitivity to anorexiant effects of leptin
