Pharmacovigilance
The etymological root: pharmakon (Greek) means drug and vigilare (Latin) means to keep awake or alert, to keep watch.
WHO Definition: The science and activities relating to the detection, assessment, understanding and prevention of adverse effects or any other drug related problems.
Why Pharmacovigilance?
- Safety data from pre-clinical studies not reliable
- Clinical trials are limited in time and number of patients
- Rare or delayed serious reactions are likely to remain unnoticed.
- To promote rational use of medicines
Disasters:
- Thalidomide in 1962
- Rofecoxib-Voluntary withdrawal (Sept 2004)
- Rosiglitazone – banned in 2010
Incidence of hospital admissions related to adverse drug reactions
- In Australia between 2.4% and 3.6%
- In United States between 3.1% and 6.2%
- In India – data not available
Hence is responsible for huge expenditure on health.
Aims of the Pharmacovigilance:
- Contribute to the regulatory assessment of benefit, harm, effectiveness and risk of medicines, encouraging their safe, rational and more effective (including cost effective) use.
- Improve patient care and safety in relation to use of medicines and all medical and paramedical interventions.
- early detection of unknown safety problems
- detection of increases in frequency
- identification of risk factors
- quantifying risks
- communicating information
- preventing patients from being affected unnecessarily
Terms in Pharmacovogilance
- Side Effect: Any unintended effect of a pharmaceutical product occurring at doses normally used in man which is related to the pharmacological properties of the drug. e.g. sedation with anti-histaminics.
- Adverse Event / Adverse Experience: Any untoward medical occurrence that may present during treatment with a pharmaceutical product at the same time, does not necessarily have a causal relationship with this treatment
- Adverse Reaction: A response to a drug which is noxious and unintended, and which occurs at doses normally used in man for the prophylaxis, diagnosis, or therapy of disease, or for the modification of physiological function
- Unexpected Adverse Reaction: An adverse reaction nature and severity of which is not consistent with domestic labelling or market authorisation, or expected from characteristics of drug action.
- Serious Adverse Event or Reaction
A serious adverse event or reaction is any untoward medical occurrence that at any dose:
- results in death
- requires inpatient hospitalisation or prolongation of existing hospitalisation
- results in persistent or significant disability/incapacity
- is life-threatening
- Congenital anomalies
Individual Case Safety Report (ICSR)
A report that contains ‘information describing a suspected adverse drug reaction related to the administration of one or more medicinal products to an individual patient’
Causality assessment
The evaluation of the likelihood that a medicine was the causative agent of an observed adverse reaction. Causality assessment is usually made according established algorithms.
SUSAR (Suspected Unexpected Serious Adverse Reaction):
Serious adverse drug reaction (SAR) that is unexpected or for which the development is uncommon (unexpected issue) observed during a clinical trial and for which there is a relationship with the experimental drug, whatever the tested drug or its comparator.
WHO Programme for International Drug Monitoring:
- WHO Drug Monitoring Programme was established in 1968
- As of October 2013, 117 countries have joined the WHO Programme for International Drug Monitoring and 30 ‘associate members’ are awaiting full membership.
It consists of a network of the
1. National Centers,
2. WHO Headquarters and
3. Uppsala Monitoring Centre which is WHO Collaborating Centre for International Drug Monitoring, in Uppsala, Sweden.
Functions:
- Identification and analysis of new adverse reaction signal.
- Provision of the WHO database.
- Information exchange between WHO and National Centers mainly through Vigimed.
- Publication of periodical newsletters, guidelines and books.
- Provision of training and consultancy support to National Centres.
- Annual meetings for representatives of National Centres.
- Producing WHO Drug Dictionary and the WHO Adverse Reaction Terminology.
- Development of pharmacovigilance as a science.
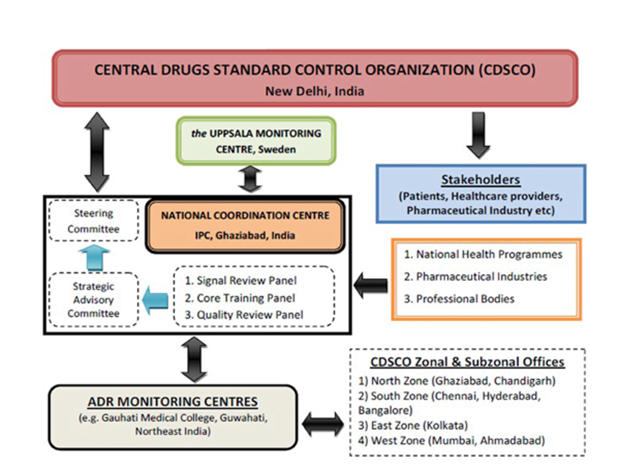
Pharmacovigilance Programme of India (PvPI):
- India joined the WHO Programme in 1998
- The Central Drugs Standard Control Organization (CDSCO) launched the National Pharmacovigilance Programme in November 2004.

- Basic purpose of this programme is to collate, analyse and archive adverse drug reaction data for making regulatory decisions regarding drugs marketed in India.
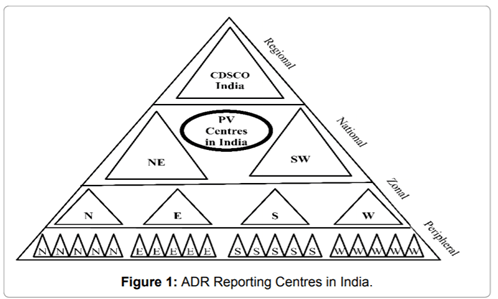
National Pharmacovigilance Program:
- The program has a three-tier structure with
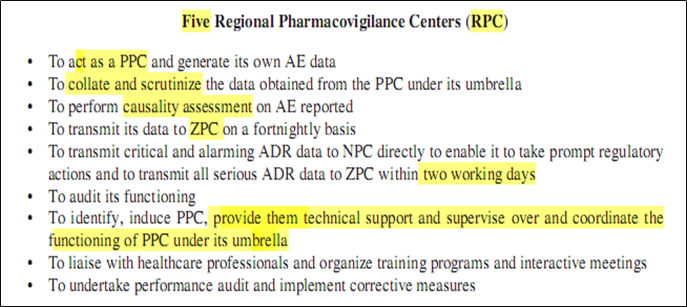
- Zonal Pharmacovigilance Centers (ZPC)
- 5 Regional Pharmacovigilance Centers (RPC)
- 26 peripheral Pharmacovigilance Centers (PPC)
- National Pharmacovigilance Advisory Committee
- National Pharmacovigilance Centre (NPC) at CDCSO



ADR Reporting
Telephone
Helpline No. – 1800 180 3024
Timing: 9 A.M to 5.30 P.M
E Mail : [email protected]
ADR Reporting – Android Apps
- Launched on 22nd May, 2015
- Joint venture by IPC, NCC and NCSB Medical College, Jabalpur
- Has an in-built algorithm for causality assessment
- Instantaneous submission
- PDF copies of duly filled ADR forms sent to the reporter’s email id and to the preferred AMC for analysis, record- keeping and entry into Vigiflow
Pharmacovigilance Process:
- Data collection & Data management
- Signal detection
- Risk assessment and quantification
- Benefit/ Risk assessment
- Actions to reduce risk or increase benefit
- Communication of risks or interventions
- Audit
- Data collection & Data management:
1. Passive surveillance
a) Spontaneous reporting
b) Case series
c) Large linked administrative database
d) Electronic medical records
2. Intensified reporting
3. Active Surveillance
4. Comparative Observational Studies
5. Targeted Clinical Investigations
6. Descriptive studies
1. Passive surveillance:
a) Spontaneous reporting
- Play a major role in the identification of safety signals once a drug is marketed.
- Identification of signals of new, rare and serious ADRs of drugs
- Most useful
1. Where reaction is unusual and unexpected
2. Where ADRs occur in close temporal relation with start of treatment or increase in dose.
- Underreporting is an important limitation.
b) Case series:
- Provide evidence of an association between a drug and an adverse event.
- More useful for generating hypotheses than for verifying an association between drug exposure and outcome
- Reports of events like TEN, SJS, Anaphylaxis should undergo detail and rapid follow up
c) Large linked administrative database:
- Large linked health administrative databases, such as Medicaid in the USA and the Ontario provincial databases
- contain data on millions of subjects
- the completeness of details, such as diagnoses, are questionable in many circumstances
- may not be representative of the whole population.
d) Electronic medical record (EMR):
- Large number and detail of the variables are available like use of tobacco products and nonprescription drugs, symptoms and signs, laboratory data and social circumstances etc.
- can be combined to generate new diagnoses or adverse events,
- Hypotheses which are not restricted to existing diagnoses, can be explored
2. Intensified reporting:
- To encourage and facilitate reporting for new products or for limited time periods methods used are
- On-line reporting of adverse events.
- Systematic stimulation of reporting of adverse events based on a pre-designed method.
3. Active monitoring:
- Patients might be identified from electronic prescription data or automated health insurance claims.
- A follow-up questionnaire can then be sent to each prescribing physician or patient at pre-specified intervals to obtain outcome information.
- More detailed information can be collected
- Efficient for those drugs used mainly in institutional settings such as hospitals, nursing homes
- Limitation – poor response rates
- Registries
- A registry is a list of patients presenting with the same characteristics
4. Comparative Observational Studies:
- Are key component in the evaluation of adverse events.
- Cross- sectional studies- primarily used to gather data for surveys or for ecological analyses
- Case-control studies- used to investigate whether there is an association between a drug (or drugs) and one specific rare adverse event, as well as to identify risk factors for adverse events
- Cohort studies- incidence rates of adverse events in addition to the relative risks of adverse events are calculated
5. Targeted Clinical Investigations:
- To elucidate the benefit-risk profile of a drug outside of the formal/traditional clinical trial setting
- To fully quantify the risk of a critical but relatively rare adverse event
- to evaluate the mechanism of action for the adverse reaction
- Pharmacodynamics and pharmacokinetic studies
- to investigate potential drug-drug interactions and food-drug interactions.
6. Descriptive studies:
- Not for the detection or verification of adverse events associated with drug exposures
- Used to obtain the background rate of outcome events and/or establish the prevalence of the use of drugs in specified populations
- Natural history of disease
- Drug Utilization Study
- Signal detection:
- Reported information on a possible causal relationship between an adverse event and a drug, the relationship being unknown or incompletely documented previously.
- Usually more than a single report is required to generate a signal, depending upon the seriousness of the event and the quality of the information.
- cannot be regarded as definitive
- indicates the need for further enquiry or action
- Signal detection pattern may be affected by coding systems and retrieval
- Signal prioritization is needed as detailed evaluation using all relevant data is complex and resource intensive
- Potential impact of a safety issue on public health is major determinant for prioritization
- SNIP criteria – Strong signals that are judged to be New, clinically Important, and have potential for Prevention will be given priority for further evaluation.
Periodic Safety Update Report
- Manufacturers submit reports to the national regulatory authority in the form of PSUR
- Includes information of all ADRs collected
- Includes evaluation of risk benefit balance
- Summarizes the market authorization status in different countries and any significant variations related to safety
- Indicates whether changes should be made to product information in order to optimize the use of the product
- In India, 6 months for first two years then annually for the next 2 years
Tools for Pharmacovigilance
- MedDRA – Medical dictionary for Drug Regulatory Activities
- It is the adverse event classification dictionary endorsed by the International Conference on Harmonisation
- WHO – ART à Coding of Adverse Events
- WHO Drug Dictionary
- Vigiflow –
- Web-based Individual Case Safety Report (ICSR) management system
- Designed for use by national centres in the WHO Programme for International Drug Monitoring
- VigiFlow is based on and compliant with the ICH E2B standard and maintained by the UMC in Uppsala, Sweden
- Vigibase – WHO Global ICSR database
- Consists of reports of adverse reactions received from member countries since 1968
Causality assessment
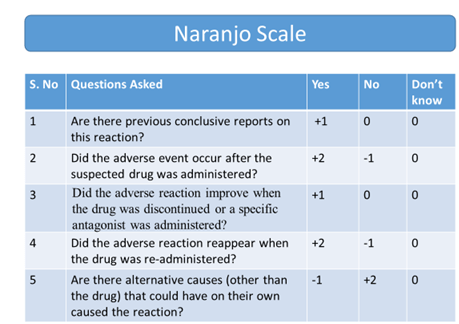
- Naranjo Algorithm
- WHO – UMC Causality Assessment – Certain/Probable/Possible/Unlikely/ Unclassified/Unassessable
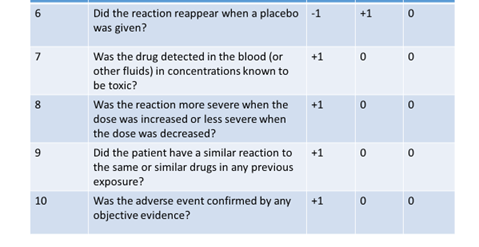
- Definite if the overall score is 9 or greater
- Probable for a score of 5-8
- Possible for 1-4
- Doubtful if the score is 0
- Benefit/ Risk assessment and decision making:
- Benefit/ Risk assessment is continued throughout life of a drug.
- Guidelines to assess risk/ benefit differ from country to country.
- CIOMS has set working group to produce guidelines on standardized Benefit/ Risk assessment.
- Key elements of Benefit/ Risk assessment:
- Description of target disease, populations being treated, purpose of intervention.
- Degree of efficacy, presence of alternative therapy.
- Type of risk and identification of risk factors.
- Consideration of all benefits and risks by indication and population.
Haemovigilance Programme of India
- Planned jointly by IPC and National Institute of Biologicals (NIB).
- National Coordinating Centre in NIB, NOIDA
- Launched on 20th December 2012 in 90 medical centres
- International Haemovigilance Network (IHN) accepted India’s application for membership in November 2015
- “A set of surveillance procedures covering the whole transfusion chain, intended to collect and assess information on unexpected or undesirable effects resulting from the therapeutic use of labile blood products and to prevent the occurrence or recurrence of such incidents.”
- Monitor transfusion reactions (Recipient Hemovigilance)
- 2296 reports in database till December 2015
Materiovigilance Programme of India
- Approval of MvPI to monitor the safety of medical devices in the country .
- MvPI formally launched on 06th July 2015 at IPC, Ghaziabad by DCG(I).
- IPC functions as National Coordination Centre.
- Sree Chitra Tirunal Institute of Medical Sciences & Technology (SCTIMST) as National Collaborating Centre, Trivandrum Kerala
- National Health Systems Resource Centre (NHSRC) under MoHFW, Govt. of India as technical support and resource centre.
Collaboration With Adverse Events Following Immunization
NCC-PvPI is assisting with adverse events following immunization (AEFI) at Immunization Technical Support Unit (ITSU) which has been set up by MoHFW with Public Health Foundation of India to ensure the vaccine safety.
Integration of Pharmacovigilance Programme of India and Revised National Tuberculosis Control Program
to improve patient care and safety in relation to the use of anti-tubercular drugs, RNTCP formally entered into collaboration with the PvPI on October 11, 2013.
Integration of Pharmacovigilance Programme of India and National Aids Control Organization
To ensure the safety of antiretroviral (ARV) medicines used in the program, IPC, NCC-PvPI, and National AIDS Control Organization formally agreed to collaborate on September 15, 2014, for setting up systems and processes for reporting, analysis, and monitoring of ADRs due to ARV medicines used in NACP.