Plasma protein binding
- Drug protein binding is the reversible interaction of drugs with proteins in plasma.
- Free drug + free protein à drug-protein complex
- Drug protein binding may be:
- Reversible: Hydrogen bonds or vander Walls forces
- Irreversible: covalent bond
- E.g.- Alkylating agents rarely
- Hepatotoxicity of acetaminophen (high doses)
Binding of drug
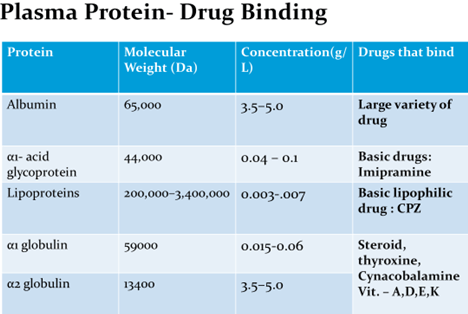
- 1) Blood components: a) plasma proteins b) blood cells
- 2) Extra vascular tissues: a) proteins b) bones c) fats
Albumin
- Each albumin molecule has at least 6 distinct binding sites for drugs and endogenous compounds.
- Two of these very tightly and specifically bind long chain fatty acids.
- There is another site which selectively binds bilirubin.
- There are two major drug binding sites called site I and site II which mainly bind acidic drugs.
- Site I binds drugs such as warfarin and phenylbutazone, whereas site II binds drugs such as diazepam and ibuprofen.
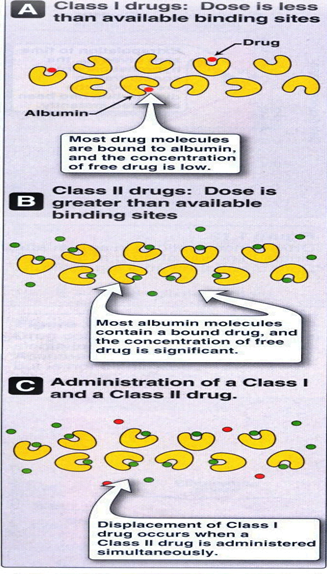
- Drugs may be categorized into two groups with respect to albumin binding:
- Class I: Drugs that have a low dose/albumin binding ratio. Albumin binding sites exceed the availability of the drug. The bound fraction consists of a significant proportion of the total drug. Many clinically useful drugs are Class I types.
- Class II: Drugs that have a high dose/albumin capacity ratio. The majority of the drug exists in the free state, bound drug is a small proportion of the total drug. Class II drugs can displace Class I drugs from albumin dramatically increasing the amount of free (active) drug.
TYPES OF DRUG-PROTEIN BINDING
- Reversible
- Irreversible eg.Chemical carcinogenesis Acetaminophen hepatotoxicity
Drug Displacement
- Competitive –drug bind to same site
- Non-competitive-inhibitory drug causing conformational change in protein molecule that inhibit binding of first drug
- Drug acting as a displacing agent has to be present in1)high conc. 2)high affinity to protein
- Acidic drugs do not displace basic drugs & vice versa.
Binding of Drugs to RBC
- Lipophilic molecules dissolved in the lipid material of the RBC membrane
- Anions can be attracted to and enter the positively charged pores of RBC
- Lipophilic drugs may be absorbed to RBC membrane due to change of:
- Change of shape of membrane and membrane proteins
- Membrane extension which may lead to change of RBC shape
The RBC binding sites are:
- Intracellular proteins
- Hemoglobin
- Carbonic anhydrase
- Cell membrane
- ATPase
Binding of drug to blood cells
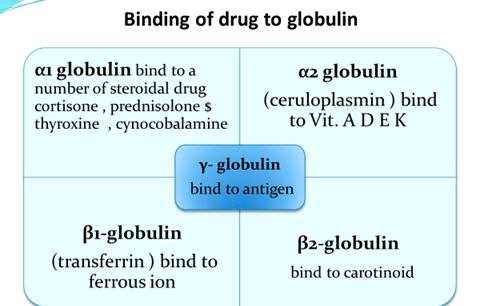
- hemoglobin – bind to phenytoin, pentobarbital , phenothiazine
- carbonic anhydrase- drug bind like acetazolamide , chlorthalidone
- cell membrane – imipramine , chlorpramazine bind to RBCs cell membrane
The Pharmacokinetic Importance of Protein Binding
- Drug-protein binding influences the distribution & equilibrium of the drug.
- Plasma proteins exert a buffer and transport function in the distribution process.
- Only free drug can leave the circulatory system and diffuse into the tissue.
- When free drug is eliminated by the body, some bound drug is released from protein binding.
- Some drugs persist in the body for three days by this mechanism.
- 2 drugs given concurrently & highly bound to the same site on a plasma protein will compete for the binding site resulting in a greater proportion of free drug.
- This effect may increase the freedrug totoxic levels.
Binding & interaction of drugs to protein
Binding of drugs may :
- Facilitate the distribution of drugs
- Inactivate the drug by not enabling a sufficient concentration of free drug to develop at a receptor site
- Retard the excretion of a drug
Interaction of drugs may cause:
- Displacement of body hormones or co-administered agent
- Change the configuration of protein to another structure capable of binding a co-administered agent
- Inactivates the drug biologically by forming a drug-protein complex
- Many drugs bind to the same receptor site but drugs with higher affinity will replace those drugs with lower affinity by competition
- Only free and unbound drugs exert therapeutic effect by interacting with receptors
Factor affecting drug protein binding
1. Factors relating to the drug
- Physicochemical characteristic of drug
- Concentration of drug in the body
- Affinity of drug for a particular protein.
2. Factor relating to the protein
- Physicochemical characteristic of the protein
- Concentration of protein
- No. of binding site on protein
3. Drug interaction
4. Patient related factor
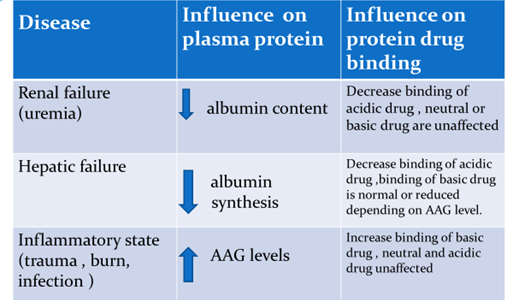
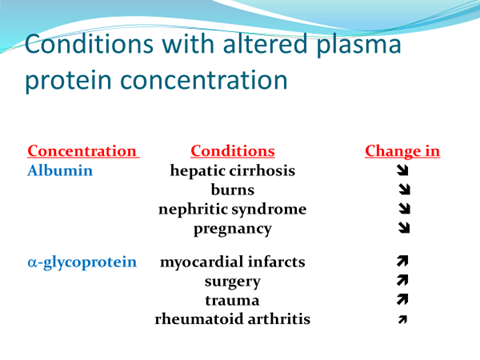
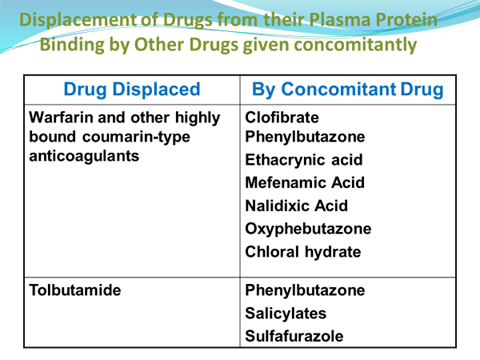
Displacement of drugs from Protein Binding is due to:
- Total amount of protein-bound drug in that body
- Extent of tissue binding structure
- Apparent volume of distribution
Methods for studying Drug-Protein binding
- Equilibrium dialysis
- Dynamic dialysis
- Diafiltration
- Ultrafiltration
- Ultracentrifugation
- Gel chromatography
- Spectrophotometry
- Electrophoresis
- Optical rotatory dispersion and circulatory dichroism
the most suitable in-vitro experimental methods are equilibrium dialysis, ultracentrifugation, and ultrafiltration. The concentration of drug in saliva,tears or the erythrocyte/plasma drug concentration ratio may give a useful measure of in-vivo binding of selected drugs-for example, phenytoin. Unfortunately, none of these methods is suitable for large-scale routine use at present.
Regulatory Guidelines
- Before initiating human trials: “In vitro” PPB data for animals and humans should be evaluated
- If extensively protein bound, the risk of displacement of other drugs should be investigated
Examples related to protein binding of drugs
- Aspirin acetylates the lysine residue of albumin, which changes the binding capacity of this protein for anti-inflammatory drugs.
- In infants, displacement of bilirubin by sulfonamides cause kernictirus
- Digoxin binds to cardiac tissues of heart. Quinine causes rise in steady state plasma level of digoxin due to displacement of tissue bound digoxin
- Warfarin, when co-administered with phenylbutazone, causes bleeding due to displacement reaction.
- Diazepam is considered restrictively eliminated while propranolol non-restrictively eliminated.
- Plasma protein binding acts as a carrier mechanism to hasten drug elimination e.g. excretion of penicillin, metabolism of lignocaine.
