Recent Advances in the Management of Asthma
- Asthma is a chronic lung disease characterized by inflammation and narrowing of the airways causing recurring periods of wheezing, chest tightness, shortness of breath, and coughing.
- Worldwide, the economic costs associated with asthma are estimated to exceed those of TB and HIV/AIDS combined
- India accounts for 10% of the asthma cases
Need for newer drugs
- Concern about the long-term safety of ICS and that they must be taken by inhalational route
- Poor adherence with ICS
- Concern about the long-term safety of LABAs, although when administered in combination with ICSs there does not seem to be a problem
- Approximately 5 to 10% of patients are not controlled despite taking effective inhaled therapy
- Different phenotypes or endotypes of asthma
- The types of new drugs needed for asthma include new classes of drug that are –
- Effective in severe, poorly controlled asthma;
- An oral treatment that is as effective as ICSs but without any side effects;
- Drugs that modify the course or even cure the disease.+
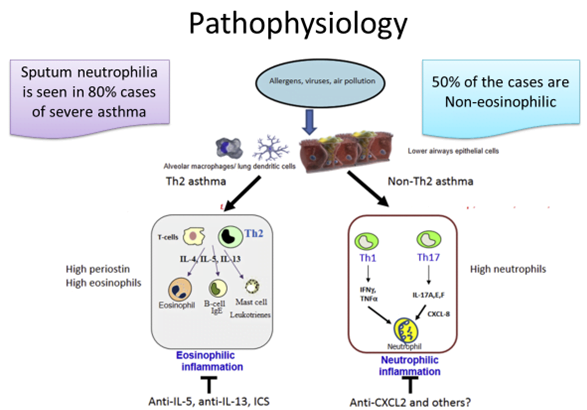
- There are at least 2 major types of asthma—Th2-high and Th2-low—dependent on the presence of selected Th2 molecular signatures
- periostin and high levels of sputum and blood eosinophils in response to Th2 cytokines such as IL-4, IL-5, and IL-13.
- Asthmatic patients with the uncontrolled severe disease despite high doses of ICSs and having high eosinophils à respond to anti–IL-5 and anti–IL-13 therapy.
- There is a negligible clinical response to anti–IL-1, anti–IL-4, anti–IL-17, or TNF-α treatment.
- Non-Th2 patients à more mixed lymphocyte profile involving Th1 and Th2 cells.
- respond to anti-CXCR2 antagonists but not to anti–IL-1, anti–IL-8, or TNF-α treatment. Studies using anti–IL-17 or other antineutrophil approaches are yet to be completed.
Th 2 Mediated Asthma
Anti IL-5 Ab
- Interleukin (IL)-5 is specifically focused on the development, differentiation, recruitment, activation, and survival of eosinophils
- Approximately 50% of severe asthma exacerbations are eosinophilic in nature
Mepolizumab (2015) and Reslizumab (2016)
- Reduce exacerbation rates and eosinophil counts in both blood and sputum
- Mepolizumab reduces oral steroid requirement by 50%, exacerbations by 50 %, given once a month
Benralizumab (Nov 2017)
- Add-on maintenance treatment of patients with severe asthma aged 12 years and older, and with an eosinophilic phenotype
- FDA approval based on the Phase III program demonstrating up to 51% reduction in asthma exacerbations, significant improvement in lung function and a 75% reduction in daily oral steroid use.
Anti IL-13 Ab
- IL-13 à mediate airway hyperresponsiveness, inflammation, mucus hypersecretion, and subepithelial fibrosis.
- Interleukin-13 à induces bronchial epithelial cells to secrete Periostin à Can be used as a biomarker for eosinophilia
- Tralokinumab failed its two Phase III trials in severe, uncontrolled asthma.
- Lebrikizumab failed to show efficacy in two Phase III studies.
- May not be sufficient to provide clinically meaningful improvements in reducing asthma exacerbations.
IL-4 Targeted Pathway
Pitrakinra
- A soluble IL-4 receptor (sIL-4R) that acts as an IL-4R antagonist is in Phase II
Dupilumab
- Fully humanized anti–IL-4Ra Mab
- Reduced asthma exacerbations in moderate-to-severe asthma, and who had a blood eosinophil count >300 cells/mL or a sputum eosinophil level >3%.
- Improved lung function and reduced levels of Th2-associated inflammatory markers. (Liberty Asthma Quest Trial)
Prostaglandin D2 receptor antagonist
- Chemoattractant receptor–homologous molecule expressed on Th2 cells (CRTh2), is a high-affinity prostaglandin D2 receptor that is expressed on major human cell types that mediate asthma pathogenesis.
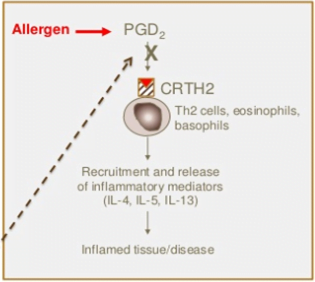
- Fevipiprant à Phase II completed
- Reduced eosinophilic airway inflammation and was well tolerated in patients with persistent moderate-to-severe asthma and raised sputum eosinophil counts despite inhaled corticosteroid treatment.
Anti – IgE antibody
Ligelizumab
- binds IgE with higher affinity than omalizumab
- Head to head comparisons suggests it may be more efficacious than omalizumab with respect to both inhaled and skin allergen responses in patients with mild allergic asthma.
- Completed phase II
Omalizumab for allergic asthma in children 6 to 11years of age (July 2016)
- Indication
- patients with moderate to severe persistent asthma and sensitization to perennial aeroallergens who are inadequately controlled on inhaled glucocorticoids
- US-FDA has now lowered the approved age range from 12 to 6 years of age.
- Black Boxed warning related to anaphylaxis has been added
- Patients should be observed for 2 hrs after the first three injections & for 30 mins after each subsequent dose because 75% of anaphylaxis cases occurred within those periods
Th 17 Mediated Asthma
- A subset of patients with severe disease that do not respond well to ICS.
- These patients may have a Th-17 predominate disease with high levels of IL-17
- Leading to more neutrophil predominance and inflammation, less reversible airflow obstruction, and less bronchial hyper-responsiveness with methacholine challenge testing.
IL- 17 Antagonists
Brodalumab,
- an anti–IL-17Rα Mab
- Despite good results in preclinical studies, had no effect on asthma control questionnaire score, FEV1, symptom scores, or a number of symptom-free days in 302 subjects with inadequately controlled moderate-severe asthma taking regular ICSs.
- Secukinumab – Phase 2 terminated
Thromboxane A2 receptor antagonist
- Thromboxane A2 (TXA2) is a bronchoconstrictor prostanoid
- Involved in the pathogenesis of acute and chronic inflammatory processes of asthma
Seratrodast
- thromboxane A2 (TXA2) receptor antagonist
- Has shown to exhibit better control on asthma than that of cysteinyl leukotriene receptor antagonists
- Global Initiative for Asthma (GINA) guidelines has recommended seratrodast as a controller of asthma in step 1 for long term management.
PDE-4 Inhibitor – Roflumilast
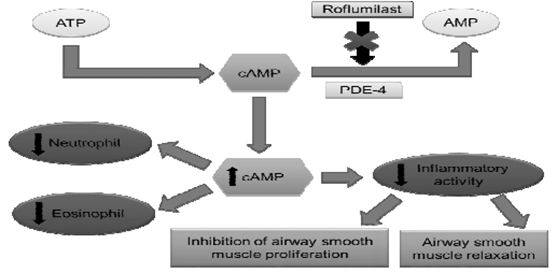
- Clinical trials were being conducted with Roflumilast for Asthma and COPD.
- Anti-inflammatory effects of roflumilast observed in COPD are also seen in asthma.
- Roflumilast attenuated allergen-induced bronchoconstriction (FEV1) in patients with asthma. Significant reductions in allergen-induced airway inflammation, including a reduction in both eosinophil and neutrophil counts, were also observed and physiologic responses to the allergen-induced challenge were confirmed by a significant reduction in TNFα. Side effects were similar to COPD but did not include weight loss.
- However, it received FDA approval only for COPD in 2011.
Bronchial thermoplasty
- Controlled radiofrequency energy is delivered to the intra-parenchymal airways during a series of bronchoscopies
- Resulting in a prolonged reduction of airway smooth muscle mass.
- Approved by FDA in 2010
- Included in step 5 of GINA guidelines
- First BT performed in India in June 2017
New strategies:
- SMART (Single inhaler maintenance and reliever therapy).
- Budesonide/formoterol is better than CS/short-acting b2 agonist.
- Rationale: ICS used as rescue therapy prevents the buildup of inflammation that precedes an acute exacerbation. Since formoterol is long-acting, even if the patient forgets a few of his maintenance dose, no harm is done. This addresses the problem of poor compliance.
New corticosteroids. (Mapracorat)
• To decrease systemic side effects
• Steroids rapidly metabolized when it enters the systemic circulation
Steroids metabolized after its action (soft steroid S/E are due to binding of steroid to DNA receptors, while anti-inflammatory effects are d/t transrepression through non-genomic effects.
• Hence dissociated steroids are being developed. (SEGRAs) Selective Glucocorticoid receptor agonist
• Limitation: Some anti-inflammatory activity might be d/t transactivation of anti-inflammatory genes.
