SERM
- About two-thirds of breast cancers express the estrogen receptor α (ER) protein.
- These ER-positive tumors are dependent on the ER and its cognate ligand, estrogen, for their growth, survival, and progression.
- Three major classes of endocrine therapy drugs, which differ by their basic mechanism of action, are in use for the treatment and/or prevention of ER-positive breast cancers.
- These therapies are all designed in one way or another to block ER function and signaling.
- Selective estrogen receptor modulators (SERMs) and
- selective estrogen receptor down-regulators (SERDs) à competitive inhibitors of estrogen binding to ERs.
- SERM/SERD hybrid (SSH) agents
- third class àaromatase inhibitors
Selective Estrogen Receptor Modulators (SERM)
Three agents are available that act as SERMs: tamoxifen, raloxifene, and toremifene
- competitive inhibitors of estrogen binding to estrogen receptors (ERs), and all have mixed agonist and antagonist activity, depending on the target tissue.
- These mixed activities have led to the redesignation of this class of compounds from “anti-estrogens” to SERMs.
The mixed antagonist/agonist effect of SERMs on ERs can be illustrated by their physiological effects in postmenopausal women:
SERMs à
- Acts as potent estrogen antagonist in breast carcinoma cells, blood vessels and at some peripheral sites,
- but as partial agonist in uterus, bone, liver and pituitary.
- Inhibition of human breast cancer cells and hot flashes reflect antiestrogenic action, while the weak estrogen agonistic action manifests as stimulation of endometrial proliferation, lowering of Gn and prolactin levels in postmenopausal women as well as improvement in their bone density.
- provide some protection against menopausal bone loss, presumably due to their partial agonist activity
- However, the increase in bone density is substantially less than that seen with estrogen therapy.
- lower serum total and low-density lipoprotein (LDL)-cholesterol concentrations
- although they do not increase serum high-density lipoprotein (HDL) cholesterol
In early cases tamoxifen is given as postmastectomy adjuvant therapy, while in advanced cases, it is a constituent of palliative treatment.
INTERACTIONS OF SERDS AND SERMS WITH THE ER
Tamoxifen —
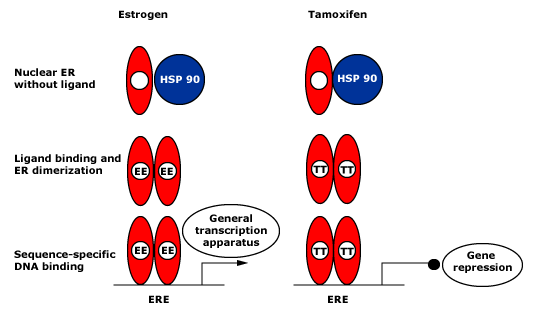
After estrogen or tamoxifen binds to the ligand-binding domain (LBD), the ER is released from heat shock protein (HSP)-90 and ER dimerization occurs.
Sequence-specific DNA binding to an estrogen-responsive element (ERE) follows. In the presence of estrogen, mRNA transcription is promoted though AF2.
Tamoxifen-bound ERs are shown as inactive, since tamoxifen inhibits AF2 function in breast cancer cells.
Raloxifene —
different tissue-specific effects from tamoxifen.
Mechanism à not fully understood
Like tamoxifen, raloxifene distorts the LBD of the ER, generating an abnormal receptor conformation that disrupts coactivator binding.
As a result, there is likely to be significant cross-resistance between tamoxifen and raloxifene, resulting in a relative lack of activity of raloxifene in tamoxifen-refractory breast cancer.
Fulvestrant, oral SERDs, and SERM/SERD hybrids —
Fulvestrant is a high-affinity competitive antagonist of ER.
Due to its similarity to the endogenous estrogen ligand, fulvestrant competes with estrogen binding to the LBD of ER. However, its long hydrophobic side chain confers fulvestrant with unique antiestrogenic properties.
Fulvestrant binding to the ER, similar to other ER ligands and SERMs, causes dissociation of the receptor from its heat-shock chaperone proteins.
However, because of its long side chain, receptor dimerization is sterically hindered and rapid proteasome-dependent ER degradation is induced
Unfortunately, fulvestrant’s poor pharmaceutical properties that prevent oral administration have limited its usefulness and potency.
Tamoxifen’s
- antagonist effect is particularly prominent with respect to breast cancer.
- Among women with ER-positive breast cancer, tamoxifen reduces the risk of recurrence and death when given as adjuvant therapy for early-stage disease and can provide palliation in those with metastatic disease
- some ER-positive breast cancers display primary resistance to tamoxifen, and all advanced breast cancers eventually become refractory to tamoxifen treatment (secondary resistance).
- may also prevent the development of contralateral breast cancer, both in women with a prior diagnosis of breast cancer and in those women at high risk of breast cancer
- only drug approved for primary as well as metastatic breast carcinoma in premenopausal women
- also effective in surgically treated cancer of male breast.
- Based on large epidemiological studies which have shown 45% reduction in the incidence of ER-positive breast cancer, tamoxifen has been approved for primary prophylaxis of breast cancer in high-risk women.
Traditionally, benefit of tamoxifen prophylaxis was considered to be limited to 5 years, but recently the ATLAS trial comparing 5 years vs 10 years of adjuvant tamoxifen, and the ‘aTTom’ study have shown
that disease free survival was higher in women who were treated for 10 years.
Side effects
Hot flashes, vomiting, rashes, vaginal bleeding. vaginal discharge and menstrual irregularities are the side effects. Increased risk of venous thromboembolism is due to estrogenic action on clotting mechanism
Toremifene
newer congener of tamoxifen with similar actions. but is a weaker ER agonist. Uses and adverse effects are also similar.
Raloxifene
- different pattern of action than tamoxifen.
- an estrogen partial agonist in bone, on lipid metabolism and cardiovascular system, but an antagonist in
- endometrium and breast.
- has high affinity for both ERa and ERβ, and has a distinct DNA target the ‘raloxifene response element ‘ (RRE).
- similar protective effect against the development of invasive breast cancer,
- lower risk of thromboembolic events and cataracts
- but a higher risk of noninvasive (in situ) breast cancer.
- Both raloxifene and tamoxifen also induce hot flashes (an estrogen antagonist effect).
- while tamoxifen clearly induces endometrial hyperplasia (an estrogen agonist effect) and increases the risk of developing endometrial cancer,
- raloxifene does not appear to have endometrioid agonistic effects; unlike tamoxifen, it does not increase the risk of uterine cancers.
Raloxifene is a second line drug for prevention and treatment of osteoporosis in postmenopausal women; Ca2- and vit D supplements enhance the benefit.
also approved for prevention of breast cancer in women with risk factors
SELECTIVE ESTROGEN RECEPTOR DOWN-REGULATORS (SERDS)
Fulvestrant
- a competitive antagonist of estrogen binding to the estrogen receptor (ER),
- only approved drug for the treatment of breast cancer that acts as an ER down-regulator.
- As opposed to the SERMs, fulvestrant is a “pure” ER antagonist with no known agonistic activity.
- proved effective in advanced breast cancer as both first-line and second-line therapy.
- approved by the US FDA for the treatment of patients with advanced ER-positive breast cancer.
- clinical efficacy in treating patients with ER-positive breast cancer, including patients who have developed resistance to tamoxifen or aromatase inhibitors (AIs)
- Unlike tamoxifen or AIs, fulvestrant has very low oral bioavailability and is administered as a monthly intramuscular injection.
- It is believed that the clinical efficacy of fulvestrant has been limited by the partial ER degradation seen in patients’ tumors as opposed to the higher degradation levels seen in preclinical breast cancer models.
●The adverse effects à in general comparable with the side effect profile of AIs, also support its systemic “pure” antiestrogenic activity.
Strong dose-dependent biological effects of fulvestrant
- Increasing the fulvestrant dose leads to reduction in ER,
- its downstream product progesterone receptor (PR), and the proliferative Ki67 marker
In patients with ER-positive metastatic breast cancer and patients with endocrine-resistant disease,
- fulvestrant combined with the cell cycle cyclin-dependent kinase (CDK 4/6) inhibitor palbociclib exerted a greater efficacy than fulvestrant alone, resulting in a markedly improved progression-free survival (PFS)
SERM/SERD HYBRIDS
- A new class of SERM/SERD hybrids (SSHs) has emerged,
- improved agonist/antagonist tissue profiles, exhibiting some of the properties of both SERMs and SERDs.
- possible future therapeutic options but remain investigational for the treatment of breast cancer at this time, pending further safety and efficacy data.
Bazedoxifene
- SSH compound that acts as an agonist in bone,
- but also effectively inhibits ER action in the reproductive system by inducing receptor degradation in these tissues.
- Bazedoxifene has been approved for the treatment of osteoporosis in Europe and, in the United States, is approved in combination with conjugated estrogen for the treatment of postmenopausal symptoms
- This compound exhibits pure antiestrogenic activity in animal models of tamoxifen-sensitive and resistant breast cancer, and has the ability to degrade ER in breast tumors.
- The combination of bazedoxifene with a cyclin-dependent kinase (CDK 4/6) inhibitor has shown therapeutic potential in models of breast tumors resistant to endocrine therapies or those expressing ESR1 mutations
