Structural Activity Relationship
- Relationship between 3D structure of a molecule and its biological/chemical activity
- Concept of linking chemical structure to a chemical property (e.g., water solubility) or biological activity including toxicity
- Identifies functional groups that are important for binding and activity
- Method – alter, remove or mask a functional group and test the analogue for activity
- Method of testing
- in vitro – for binding interactions with target (e.g. enzyme)
- in vivo – for target binding interactions + pharmacokinetic properties
- If group is removed or modified and in vitro activity:
- drops or diminished à group was important for binding
- unaffected à group is not important
Hansch Equation
- free energy related model to correlate biological activities with physiochemical properties
- In a simple situation where biological activity is related to only one such property, a simple equation can be drawn up. The biological activity of most drugs, however, is related to a combination physicochemical properties. In such cases, simple equations involving only one parameter are relevant only if the other parameters are kept constant. In reality, this is not easy to achieve and equations which relate biological activity to a number of different parameters are known as HANSCH EQUATION.
- Correlates activities with physicochemical parameters
- “Outside” predictions are possible
Objectives
- to determine as accurately as possible the limits of variation in the structure of a chemical that are consistent with the production of a specific effect (e.g., can a chemical elicit a specific toxic endpoint)
- to define the ways, which alterations in structure and thereby the overall properties of a compound influence endpoint potency
- To develop or modify a drug that has increased activity
- To develop a drug with fewer unwanted side effects
- Drug receptor interaction on the basis of various physio-chemical properties.
Need for SAR
- The selection of the changes required to produce analogues of a particular lead
- Structural changes that result in analogues with increased lipid character may exhibit either increased activity because of better membrane penetration n = 3-6) or reduced activity because of a reduction in their water solubility.
- Its effect on water solubility, transport through membranes, receptor binding, and metabolism and other pharmacokinetic properties of the analogue should be considered as far as is possible before embarking on what could be an expensive synthesis.
- Furthermore, changing the structure of the lead compound could result in an analogue that is too big to fit its intended target site.
- Computer assisted molecular modelling can alleviate this problem, provided that the structure of the target is known or can be simulated with some degree of accuracy.
- To find out which groups are essential for biological effect.
- All analogues are tested for biological activity and compared to original compound, if an analogue shows significant change in activity, that group is considered important
Basic Principles of SAR
Similar Structure and Similar Action
Adrenaline (activity at all α & β receptors) , Noradrenaline (no β2 activity)
Similar Structure and Dissimilar Action
- E.g: Noradrenaline –Vasoconstriction, no action on bronchial smooth muscle
- Isoprenaline- vasodilation & bronchodilation
Similar Structure and No Action
- E.g: molecular isomerism – dextro & levo compounds differ in activity (dextro amphetamine is much more active than levo form)
Dissimilar Structure and Similar Action
- E.g: Acetylcholine, pilocarpine, muscarine
Implications
- Increased duration of action – Acetylcholine and methacholine (Slowly hydrolyzed by AChE)
- Decreased duration of action – Atropine produces Mydriasis & Cycloplegia for a week, Homatropine produces Mydriasis & Cycloplegia for 24hrs
- Increased specificity – Structural modifications of Chlorpromazine (Anti histaminic, Anti cholinergic, Hypotensive, Anti-psychotic) à Trifluoperazine à More potent antipsychotic effect
- Reduced ADRs à Phenacetin associated with Analgesic Abuse Nephropathy à Paracetamol
- Competitive antagonist à Para amino benzoic acid (PABA) essential growth factor for many microorganisms, Sulphonamide structural similarity with PABA acts by competing with PABA for uptake by certain bacteria, Morphine–Nalorphine
SAR Applications
- SAR of Beta Lactam à modification in penicillin to form cephalosporin
- L.As with Ester linkage are hydrolysed readily by plasma esterases Procaine, Tetracaine, Benzocaine, Chlorprocaine
- Amide linkage à Not hydrolysed by plasma esterases, More intense & long lasting action, No cross sensitivity with ester Las à lidocaine, bupivacaine, prilocaine
Chirality
- Each form (left- or right-handed) of a chiral compound is called ‘enantiomer’ or ‘isomer‘
- Enantiomers are denoted as R or S or
- Dextro (+) or levo (-) depending on clockwise or anticlockwise rotation of plane-polarized light
- 50/50 mixture of the two enantiomers is called a racemic mixture or a racemate


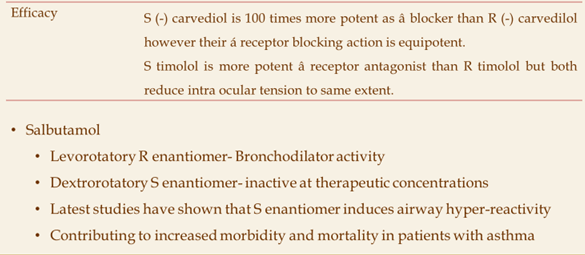
SAR and Drug Development
- Once structure of lead compound is known, next step is to study its SAR.
- Aim is to discover which parts of the molecule are important to biological activity and which are not.
- SAR is synthesizing compounds, where one particular functional group of the molecule is removed or altered.
- In this way it is possible to find out which groups are essential for biological effect.
- All analogues are tested for biological activity and compared with original compound
- If an analogue shows significant change in activity, that group is important
Various Approaches for Drug Development
- Ligand–‐based
- (Quantitative) structure – activity relationship (SAR & QSAR) à Molecular docking
- Bioinformatics (target recognition and structural modelling)
- Gene identification and prediction
QSAR
- QSAR approach attempts to identify and quantify the physicochemical properties of a drug and to see whether any of these properties has an effect on the drug’s biological activity
Considerations for QSAR
- All compounds should belong to congeneric series
- Similar mechanism of action
- A similar binding mechanism
- Biological activity should be exactly the same
- Biological activity is correlated to binding affinity
- Purpose of QSAR
- To predict biological activity and physico-chemical properties by rational means
- To comprehend and rationalize the MOA within a series of chemicals
- Savings in the cost of development
- Reduction in requirements of lengthy and expensive animal tests
- Other areas of promoting green chemistry to increase efficiency and eliminate waste
Applications of QSAR
- Development of new congeners with
- ↑/↓ duration of action
- Restricted action to particular system of body
- Reduced adverse drug reactions & toxicity
- Drugs with better pharmacokinetic profiles can be developed
- Understanding mechanism of action of drugs
- Lead compound optimization
- Understanding molecular mechanisms of drug resistance and circumventing it
- Environmental chemistry
