Malaria
As per latest WHO estimate.* ~ 2 I 6 million cases and ~ 0.445 million deaths occurred globally due to malaria in 2016, out of which 90% cases and deaths were in Africa and 7% in south-east Asia (including India).
National vector borne diseases control programme’ (NVBDCP) 2016à reported 1.06 million confirned malaria cases in India, out of which nearly 50% were falciparum malaria.
Quinineà isolated from Cinchona bark
Clinical manifestation
- Cardinal signs and symptoms of malaria are high, spiking fevers (with or without periodicity), chills, headaches, myalgias, malaise, and GI symptoms.
- Headache, a characteristic early symptom in malaria caused by all Plasmodium spp.
- Tertian malaria: fever every third day (first day is number 1): P. vivax and P. ovale
- Subtertian (malignant tertian) malaria: fever slightly more frequent than every third day: P. falciparum
- Quartan malaria: fever every fourth day: P. malariae
Microscopy of stained thick and thin blood smears remains the gold standard for confirmation of diagnosis of malaria
OBJECTIVES AND USE OF ANTIMALARIAL DRUGS
The aims of using drugs in relation to malarial infection are:
• To prevent clinical attack of malaria (prophylactic).
• To treat clinical attack of malaria (clinical curative).
• To completely eradicate the parasite from the patient’s body (radical curative).
• To cutdown human-to-mosquito transmission (gametocidal).
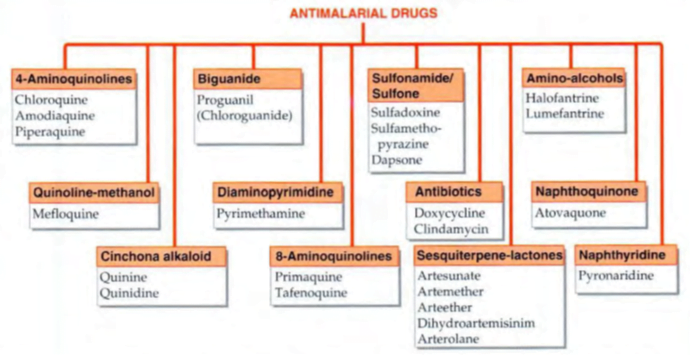
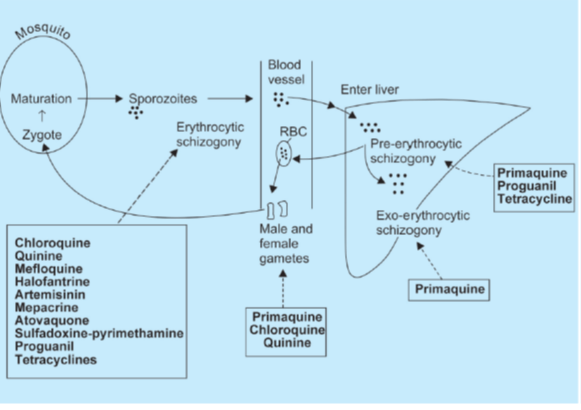
• Primary tissue schizonticides: à kill schizonts in the liver (pre-erythrocytic stage) e.g. proguanil, primaquine and pyrimethamine. These drugs are used for causal prophylaxis.
• Erythrocytic schizonticides: à kill schizonts in the blood and can be used for the treatment of acute attacks as well as suppressive prophylaxis of malaria.
used for treatment of malaria but not for prophylaxis. Artemisinin derivatives are very short acting whereas quinine and sulfadoxine are toxic on long term administration, therefore are not suitable for prophylaxis.
– Fast acting: Chloroquine, mepacrine, quinine, mefloquine, halofantrine, atovaquone and artemisinin derivatives
– Slow acting: Proguanil, pyrimethamine, sulfonamides, tetracyclines
• Exo-erythrocytic schizonticides à kill the exo-erythrocytic forms and are thus used for radical cure e.g. primaquine.
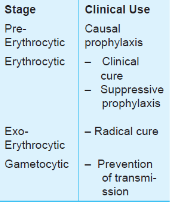
• Sporonticides or gametocides à kill the gametes and thus prevent transmission of malaria. Chloroquine, mepacrine and quinine kill the gametes of P.vivax only whereas proguanil, pyrimethamine, primaquine and artemisinin kill gametes of both P. vivax as well as P. falciparum.
General recommendations for the management of uncomplicated malaria-
● Avoid starting treatment on an empty stomach. The first dose should be given under observation.
● Dose should be repeated if vomiting occurs within half an hour of antimalarial intake.
● The patient should be asked to report back, if there is no improvement after 48 hours or if the situation deteriorates.
● The patient should also be examined and investigated for concomitant illnesses.

Mixed P. falciparum and P. vivax infection: à treated as falciparum malaria with ACT other than artesunate – sulfa-pyrimethamine, because sulfa-pyrimethamine is not effective against P vivax.
Anti-relapse treatment with 14-day course of primaquine may be started concurrently.
Falciparum malaria during pregnancy àtreated promptly and aggressively. Drugs recommended are :
I. Quinine 600 mg TDS x 7 days + clindamycin 300 mg TDS/QID (20 mg/kg/day) for 7 days; can be used during all trimesters, and is a safer option during 1 st trimester.
2. Artemisinin-based combination therapy à better tolerated 3 day regimen, which may be used during the 2nd and 3’d trimester in preference to 7 day quinine + clindamycin therapy. Safety of ACT during I st trimester is unproven.
Vivax malaria during pregnancy àtreated with chloroquine as in non-pregnant, but radical cure with primaquine should be postponed till after delivery.
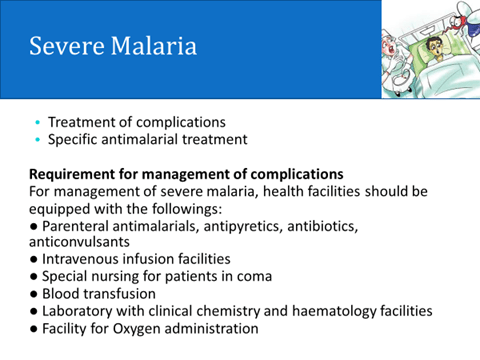
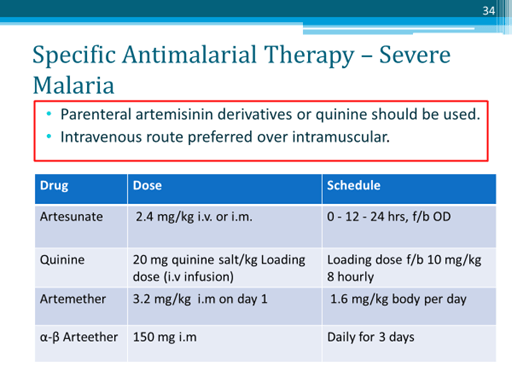

Severe and complicated falciparum malaria àP falciparum infection attended by any one or more of-
hyperparasitaemia, hyperpyrexia, fluid and electrolyte imbalance, acidosis, hypoglycaemia (blood glucose < 40 mg/dl), prostration, cardiovascular collapse, jaundice, severe anaemia, spontaneous bleeding, acute respiratory distress, pulmonary edema, haemoglobinuria, black water fever, renal failure and cerebral malaria (impaired consciousness/convulsions/coma).
Radical cure
- About 8- 30% P.v. cases relapse due to persistance of exoerythrocytic stage.
- A radical curative is needed in relapsing malaria, while in falciparum malaria adequate treatment of clinical attack leaves no parasite in the body (there is no secondary exoerythrocytic tissue phase).
Drug of choice for radical cure of vivax and ovale malaria is:
• Primaquine 15 mg daily for 14 days given concurrently with or immediately after chloroquine/other schizontocide
- test negative for G-6- PD deficiency.
- Tafenoquine, a new long-acting 8-aminoquinoline exoerythrocytic schizontocide, has been developed as a single dose antirelapse drug for vivax mala ria
which is awaiting approval.
Gametocidal à refers to elimination of the male and female gametes of Plasmodia formed in the patient’s blood. Gametocidal action is of no benefit to the patient being treated,
but will reduce transmission to mosquito.
Primaquine à gametocidal to all species of Plasmodia, while artemisinins have weak lethal action on early-stage but not mature gametes.
• A single dose of primaquine is employed immediately after clinical cure of falciparum malaria to kill the gametes and cut down transmission to mosquito.
- In India, the dose of primaquine for this purpose recommended by NBVDCP is 45 mg (0.75 mg/kg), while
- WHO (20 15) recommends 15 mg (0.25 mg/kg), which is considered safer for mass use,and sufficient to interfere with transmissibility to mosquito.
Suppressive prophylaxis
Chloroquine sensitive P.f à
Chloroquine (CQ) 300 mg or 5 mg/kg weekly.
In travellers. start one week before with a loading dose of 10 mg/kg and continue till one month after return from endemic area.
Since CQ-resistant Pf is now widespread in Indiaà CQ is no longer employed as prophylactic in India.
- Short-term chemoprophylaxis (less than 6 weeks)
Doxycycline: 100 mg daily in adults
1.5 mg/kg body weight for children more than 8 years old.
Drug started 2 days before travel and continued for 4 weeks after leaving the malarious area.
- It is contraindicated in pregnant women and children < 8 yr.
- Long-term chemoprophylaxis (more than 6 weeks)
Mefloquine: 5 mg/kg body weight (up to 250 mg) weekly and
should be administered 2 weeks before, during and 4 weeks
after leaving the area.
- In India, use of mefloquine for prophylaxis is not allowed among residents, but may be used by travellers for long term (6 weeks to I year) prophylaxis.
Chemoprophylaxis à
- limited to short-term use in special risk groups, such as – nonimmune travellers,
- nonimmune persons living in endemic areas for fixed periods (army units, labour forces), infants, children and pregnant women (falciparum malaria has serious consequences in the pregnant).
Chloroquine
- drug possessing largest volume of distribution (>1300 L)
- accumulates in the food vacuole of the plasmodium à selectively concentrated in the parasitized erythrocytes.
- prevents polymerization of heme to hemozoin resulting in accumulation of heme that is toxic for the parasite
- drug of choice for treatment and prophylaxis of non-falciparum malaria and chloroquine sensitive P. falciparum malaria.
- an erythrocytic schizonticide and has no effect on exo-erythrocytic stages.
- also used for other indications that are
• Rheumatoid arthritis
• Extraintestinal amoebiasis
• Discoid lupus erythematosis
• Lepra reaction
• Infectious mononucleosis
• Photogenic reactions
• Malaria
• Giardiasis
Adverse effects of chloroquine
skin rashes (lichenoid eruptions), peripheral neuropathy, hypotension, myocardial depression (T wave changes in ECG), auditory impairment and toxic psychosis.
Prolonged use of high doses can result in blindness due to retinal damage (Bull’s eye maculopathy). It can also precipitate porphyria and cause discolouration of nails and mucous membranes.
Chloroquine is the drug of choice for treatment of malaria in pregnant women.
Quinine
- mechanism of action is not clear and may be similar to chloroquine.
- major use is treatment of P. falciparum infections resistant to chloroquine (drug of choice).
- often used with doxycycline or clindamycin to decrease the duration of therapy and limit toxicity.
- To delay emergence of resistance, it is not advocated for chemoprophylaxis.
- It is 70% bound to plasma proteins especially α1 acid glycoprotein, such binding increases in acute attacks of malaria, so that patients of malaria can tolerate much higher doses of quinine than other subjects.
- Its d-isomer, quinidine can be used i.v. for severe P. falciparum infections.
- It can cause hypoglycemia manifested by palpitations, sweating and tachycardia.
- To prevent hypoglycemia, i.v. infusion of quinine should always be given in 5% dextrose solution (instead of normal saline).
- At toxic doses, cinchonism can occur which manifests as symptoms of gastrointestinal distress, vertigo, blurred vision, headache and tinnitus. It can also cause cardiac conduction abnormalities and hemolysis in G-6-PD deficient patients.
- According to WHO guidelines, quinine is safe in pregnancy and can be used for severe or chloroquine resistant malaria.
Mefloquine
- can be used for chloroquine resistant P. falciparum infections, both for treatment as well as prophylaxis.
- It can cause cardiac conduction disturbances, psychosis and seizures.
- Administration with halofantrine or quinine is contraindicated because it can cause prolongation of QT interval.
- effective as a single dose treatment of malaria.
Primaquine
- acts by forming redox compounds that act as cellular antioxidants.
- It is a tissue (pre as well as exo-erythrocytic) schizonticide and gametocide.
- It is always used along with blood schizonticides for radical cure of malaria.
- It can cause methemoglobinemia and hemolysis in G-6- PD deficient patients.
- It is contra-indicated in pregnancy.
- It has no role (for radical cure) in P. falciparum malaria because this organism has no exo-erythrocytic stage.
Antifolate Drugs
- include pyrimethamine, proguanil, sulfadoxine and dapsone.
- Proguanil is a prodrug and is activated to form cycloguanil.
- Pyrimethamine and cycloguanil act by inhibiting DHFRase.
- Pyrimethamine plus sulfadoxine act through sequential blockade.
- slow acting blood schizonticides that are active against chloroquine resistant P. falciparum infections.
- Proguanil plus atovaquone can be used for treatment as well as chemoprophylaxis of chloroquine resistant malaria.
Atovaquone
- rapidly acting blood schizonticide that acts by collapsing the parasite’s membrane.
- Proguanil potentiates its antimalarial action.
- It can also be used for Pneumocystis jiroveci pneumonia and Toxoplasma gondii infections.
Halofantrine and Lumefantrine
- Halofantrine has erratic oral bioavailability and can cause potentially serious cardiotoxicity (even more if combined with mefloquine).
- Due to these reasons, it is not recommended for chemoprophylaxis of malaria.
- Use of this drug is reserved for treatment of multidrug resistant malaria.
- Lumefantrine is a new drug similar to halofantrine and is always used along with artemether.
- absorption markedly increases with fatty food.
Other drugs
Other antimalarial drugs include doxycycline, amodiaquine, mepacrine and pyronaridine etc.
Mepacrine is most concentrated in collagen.

Parenteral artesunate is the drug of choice in pregnancy in all trimesters.
Intermittent preventive treatment in pregnancy
- In the form of one dose pyrimethamine (75 mg) + sulfadoxine (1500 mg) each in 2nd and 3rd trimester (gap not < I month) is recommended by WHO for areas with high P .f endemic ity (P .f >30%) to protect pregnant women.
Artemisinin Derivatives
- Artemisinin, dihydroartemisinin, artesunate, artemether and arteether
- obtained from a Chinese herb Artemisia annua.
- Artemisinin is a prodrug and is activated in the body to dihydroartemisinin.
- These drugs generate highly active free radicals that damage parasite membranes.
- fastest acting drugs against malaria.
- Artesunate has a very short half life and can be given i.v.
- These can be used for the treatment of multidrug resistant malaria as well as serious forms like cerebral malaria.
- Artemisinin derivatives are not indicated for chemoprophylaxis of malaria. It can rarely cause QT prolongation.
Quinine infused i. v. had been the drug of choice for severe and complicated malaria, including cerebral malaria, but now i. v./i.m. artemisinins are preferred, while quinine is used only as an alternative when artemisinins cannot be used.
Because i. v. injection achieves more rapid peak concentration, and only artesunate sod. can be given i.v., the NVBDCP uses only i.v. artesunate for severe malaria.
In the erythrocytic schizogony cycle, artemisinins exert action on a wide range of stages- from ring forms to early schizonts; thus have the broadest time window of antimalarial action.
Artesunate
- administered by oral, i.m. or i.v. routes.
- After oral ingestion, absorption is incomplete but fast, reaching peak in <60 min.
- It is rapidly converted to the active metabolite dihydroartemisinin (DHA) with at½ of 30- 60 min. The t½ of DHA is 1- 2 hours.
- Intravenous artesunate is the I st choice drug for severe malaria.
Artemether
- lipid-soluble and is administered orally or i.m., but not i.v.
- For severe malaria, it is a 2nd choice option when i.v. artesunate is not available or cannot be used.
- Oral artemether is used in combination with lumefantrine
Arteether
- This compound developed in India is available for i.m. administration only to adults for complicated malaria.
- Because of its longer elimination t½ (23 hours), it is recommended in a 3 day schedule, but is considered less dependable in severe/complicated malaria.
- The WHO recommends a 5 day course
- Dose: 150 mg i.m. daily for 3 days in adults.
Dihydroartemisinin (DHA) and Arterolane
- available for oral use only in combination, and are considered with ACT
Adverse effects
- artesunate and artemether produce few adverse effects; most are mild:
nausea, vomiting, abdominal pain, itching, drug fever, delayed haemolysis and other hypersensitivity reactions. Headache, tinnitus, dizziness, bleeding, dark urine, transient reticulopenia and leucopenia are rare and subside when the patient improves or the drug is stopped.
Intravenous artesunate is much safer than i. v. quinine.
Interactions Concurrent administration of artemisinins with drugs prolonging Q-T, like antiarrhythmics, tricyclic antidepressants and phenothiazines may increase the risk of cardiac conduction defects.
ARTEMISININ-BASED COMBINATION THERAPY (ACT)
- Because of emergence of CQ-resistant, followed by multidrug-resistant P falciparum, the WHO has recommended that all cases or acute uncomplicated falciparum malaria should be treated only by combining one of the artemisinin compounds with another effective erythrocytic schizontocide.
- In choosing the companion drug, the most important consideration is its elimination t½ (governing stay of the drug in the body), because effective concentrations in blood must be maintained for at least 3-4 asexual cycles of the parasite, i.e. 6- 8 days, to exhaust the parasite burden.
- Therefore, short t½ drugs have to be given for 7 days, while longer acting drugs can be given for 1- 3 days.
- However, long t½ drugs allow subinhibitory concentrations to persist in the blood facilitating selection of resistant mutants.
- Combining a short t½ drug with a long t½ drug in the conventional 3 day regimen runs the risk of de facto monotherapy after the short t½ drug is eliminated.
- This risk is minimized by choosing a short t½ drug that reduces the parasite load rapidly and drastically.
- Artemisinin compounds fulfil this requirement, as they rapidly kill > 95% plasmodia.
Advantages of ACT over other antimalarials are:
• Rapid clinical and parasitological cure.
• High cure rates (>95%) and low recrudescence rate.
• Absence of parasite resistance (
• Good tolerability profile.
The ACT regimens for oral treatment of uncomplicated falciparum malaria
Oral ACTs are not to be used in severe or complicated malaria, for which parenteral drugs are needed.
1. Artemether-lumefantrine
- Lumefantrine is used only in combination with artemether, as FDC tablets.
- The two components protect each other from plasmodial resistance.
- Clinical efficacy is high achieving 95- 99% cure rate.
- Artemether-lumefantrine is active even in multidrug resistant Pf areas including MQ-resistant.
- While artemether quickly reduces parasite biomass and resolves symptoms, lumefantrine prevents recrudescence. Gametocyte population is reduced à checking transmission.
- must be administered with fatty food or milk, which markedly enhances lumefantrine (and to some extent artemether) absorption. and ensures adequate blood levels.
- Comparative studies indicate that it is better tole rated than AS/MQ.
- It is to be avoided in first trimester of pregnancy and during breastfeeding.
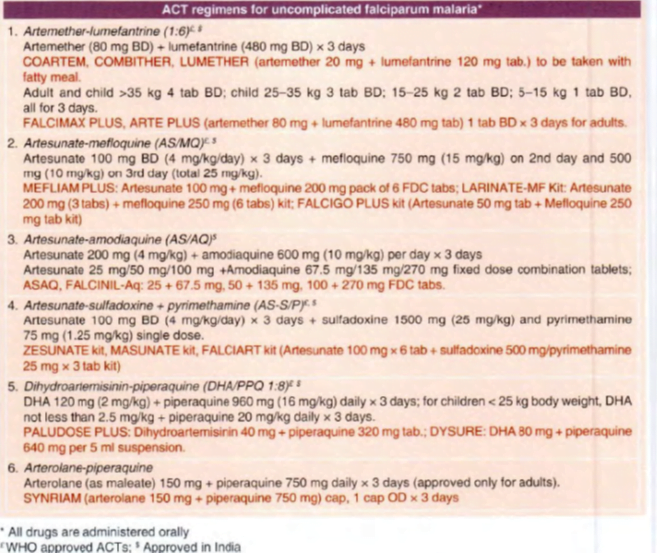
2.Artesunate-mefloquine (AS/MO)
- adverse effect potential of AS/MQ is less favourable than other ACTs.
- Therefore, it is less popular now.
- In India AS/MQ has been used lo a limited extent, but small studies have shown – I 00% efficacy.
3. Artesunate-amodiaquine(AS/AQ)
This ACT has now been marketed in India as FDC tablets in 3 strengths for different age groups
to be taken twice daily for 3 day treatment of uncomplicated falciparum malaria.
4. Artesunate-sulfadoxine + pyrimethamine (AS-S/P)
- This ACT has been adopted as the first line treatment for uncomplicated falciparum malaria in India, except in the north-eastern states bordering Myanmar, from where S/P resistance has been reported.
- This does not imply that AS-S/P is the best ACT because it is not effective against multi drug resistant strains which are nonresponsive to S/P.
- However, in rest of India so far this ACT appears to be working satisfactorily with >96% success rate.
- As such, NVBDCP continues to use AS-S/P ACT as the first line therapy, including that during 2nd and 3rd trimester of pregnancy.
- Private clinics, however, are using alternative ACTs.
5. Dihydroartemisinin (DHA)-piperaquine
- Piperaquine – mechanism of action is similar to CQ.
- It is more active against CQ-resistant P falciparum and retains equal activity against CQ sensitive organisms.
- efflux transporters of the resistant parasite are believed to be unable to pump out the bulky molecule of piperaquine.
- Piperaquine is coformulated with DHA in a dose ratio of 8: I
- Safety profile of DHA-piperaquine is good and it is well tolerated even by children.
- The DHA-piperaquine FDC has achieved >98% response rate in clinical trials on uncomplicated falciparum malaria patients in India, and has been approved.
6. Arterolane-piperaquine
- Arterolane à developed in India and marketed in combination with piperaquine.
- Arterolane acts rapidly at all stages of asexual schizogony of malarial parasite including multidrug resistant Pf, but has no effect on the hepatic stages.
- It accumulates in the food vacuole of the parasite, and thus differs from artemisinins which do not accumulate at this site.
- absorbed orally, and absorption is unaffected by food.
- In uncomplicated falciparum malaria this ACT has produced 95% cure rate with a fever and parasitaemia clearance time of 24-48 hours.
- Side effects are generally mild headache, postural dizziness, vomiting, abdominal pain and diarrhoea.
7. Artesunate-pyronaridine
- Pyronaridine à resembling structurally to amodiaquine.
- It is an erythrocytic schizontocide with high efficacy and mechanism of action similar to CQ.
- The onset of action is slower and duration long.
- It is concentrated in RBCs and metabolized with a terminal t½ of 7 days.
- Weak analgesic-antipyretic action is produced al higher doses.
- Clinical trials have been completed in India with > 95% cure rate. However. this ACT has not yet been approved for use in India.
Use
- Uncomplicated falciparum malaria
- Oral artemisinins are indicated for the treatment of all cases of uncomplicated falciparum malaria (CQ-resistant as well as sensitive) as ACT.
- Even when used alone, they are almost 100% effective, but because of short duration of action recrudescence rates are high.
- In order to preserve their powerful antimalarial activity and to reduce recrudescence rates, they must be used in combination with a long acting schizontocide which acts by a different mechanism.
- Monotherapy with oral artemisinin derivative is prohibited in India.
- For vivax malaria, artemisinins (as ACT) are indicated only in case of CQ-resistant infection and when quinine + doxycycline/clindamycin also cannot be used.
- Use of artemisinins for prophylaxis of malaria is not allowed because wide spread prophylactic use will foster resistance.
- Moreover, they have a short duration of action and higher potential toxicity.
- Their cidal action on early stage gametes reduces transmission of resistant P.f infection, but does not totally interrupt it. Single dose primaquine is recommended after ACT to kill all circulating gametes.
- Severe and complicated falciparum malaria
- Parenteral artemisinins à highly effective and are the drugs of choice irrespective of CQ resistance status.
- Quinine infused i. v. had been the drug of choice for severe and complicated malaria, including cerebral malaria, but now i. v./i.m. artemisinins are preferred, while
- Quinine is used only as an alternative when artemisinins cannot be used.
- Quinine (i. v.) continues to be the drug used for severe falciparum malaria during I st trimester of pregnancy, because safety of artemisinins is not yet proven.
Artesunate (i.v.) offers several advantages:
• It causes faster parasite clearance than i. v. quinine.
• It is safer and better tolerated than i.v. quinine.
• Its dosing schedule is simpler.
• Evidence from clinical trial indicates higher efficacy and lower mortality.
Because i. v. injection achieves more rapid peak concentration, and only artesunate sod. can be given i.v., the NVBDCP uses only i.v. artesunate for severe malaria.
Malaria Vaccine Candidates in Clinical Development
Tafenoquine – Approved July 2018
Specific Treatments:
prevention of malaria relapse in patients receiving appropriate antimalarial therapy
(tafenoquine) is an 8-aminoquinoline derivative with activity against all stages of the P. vivax lifecycle, including hypnozoites.
specifically indicated for the radical cure (prevention of relapse) of Plasmodium vivax malaria in patients aged 16 years and older who are receiving appropriate antimalarial therapy for acute P. vivax infection.
oral administration. The recommended dose in patients aged 16 years and older is a single dose of 300 mg administered as two 150-mg tablets taken together.
Recent advances in malaria
- Need for new drugs: No drugs acting on sporozoites, no drugs acting on all stages, no focus on mixed infections, Lack of efficacy of transmission blocking drugs (especially pyrimethamine reversal effect), lumefantrine absorption being fat dependent, Increased resistance to ACT, decreased compliance to primaquine l/t relapses.
- Recently approved ACT in India (ALL FROM MY NOTES)
- Artemether (20 mg)- lumefantrine(120 mg).
- 6 doses: 1 dose given BD for 3 days.
- Dose is dependent on weight. 5-15 kg: 1 tablet, 15-25 kg: 2 tablets, 25-35 kg: 3 tablets, >35 kg: 4 tablets.
- C/I in first trimester of preg and <5 years of age
- Apporved by FDA in Aug 2009.
- It had better 28 day cure rate, faster parasite clearance and faster fever clearance.
- It is the first, taste masked pediatric dispersible solution available.
- Novartis is going to submit a resistance report in 2016.
- Artesuate (100 mg BD for 3 days) and SP (1500 mg and 75 mg single dose).
- First line drug for uncomplicated P.f under NVBDCP. NVBDCP also states that the combination should be used as first line even in 2nd an 3rd trimester of preg.
- Not effective against MDR malaria
- Artesuate (100 mg BD for 3 days) & mefloquine (750 mg on day 2 and 500 mg on day 3)
- Neuropsychiatric s/e.
- Artesuate (100 mg BD for 3 days) and Amodiaquine (300 mg BD for 3 days)
- a/w agranulocytosis and neutropenia in HIv patients
- 3 different strengths are approved in India
- ACTs under clinical trials in India
- DHA (40 mg OD for 3 days)and Piperaquine (320 mg OD for 3 days): Piperaquine has a long half life of 63 days.
- Modification of structure of existing anti-malarials
- Arterolane-piperaquine combination
- MOA of Artemisinins is that the peroxide bridge in its structure is cleaved to generate reactive oxygen species. Arterolane is a synthetic artemisinin derivative.
- It is the first artemisinin like drug developed in India.
- Another advantage is that this combination does not need fat dependent absorption.
- Old drugs with new uses:
- Azithro (250 mg) and Chloroquine (155 mg): Azithromycin shows clinical synergy with chloroquine even where chloroquine resistance is as high as 50%. This combination has entered Phase III for intermittent preventive treatment of Plasmodium falciparum malaria in pregnancy (IPTp) in Oct 2010. The primary outcome is a reduction in the number of subjects with suboptimal pregnancy outcome, and results should be available in 2014. 4 tablets are to be taken in pregnancy and is the most advanced non ACT based regimen in pipeline.
- Cotrimoxazole: For intermittent preventive therapy for pregnancy. Being compared to SP and DHA-piperaquine.
- Artemisinin resistance: Atremisone with mefloquine single dose is being tested for artemisinin resistance.
- Fosidomysin: Apicoplast inside the malarial parasite is involved in protein synthesis, FA synthesis and mevalonate independent isoprenoid synthesis. Fosidomysin inhibits this mevalonate independent isoprenoid synthesis. It is in CT II as fosidomysin and clindamycin combination.
- Methylene blue and chloroquine has failed d/t lower efficacy. However MB and Artesuate/atovoquane combination is under phase 2 trial.
- Limitations of Primaquine. Long term therapy of 14 days decreases compliance and hence decreases efficacy for protection against relapse. Tefanoquine is a congener of primaquine which needs to be given for 3 days only thereby increasing compliance. This has translated into increased efficacy of 90% a/c/t 70% for 6 month relapse free period. Taking note of this US-FDA has granted Breakthrough therapy designation for accelerated approval.
- Novel targets: DHFR (it is pyrimethamine and proguanil target), Dihyroorotate dehydrogenase (It is atovaquone target), Glucose transporter inhibitors (this decreases glucose transport inside the parasite thereby decreasing ATP generation and maintenance of membrane polarity)
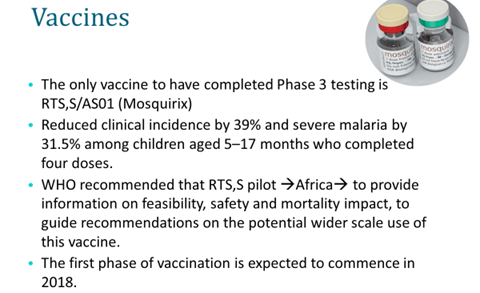
- Malarial vaccine:
- To meet the challenges the global malaria vaccine community came together to establish a shared vision and goals and to identify the activities that could address some of the above-mentioned challenges in Aug 2006 culminating in the development of Malaria Vaccine technology Roadmap. It has a strategic goal of developing a vaccine by 2020 with 80% protective efficacy against clinical disease of P. Falciparum and last longer than 4 years along with the landmark aim to develop and license a first-generation malaria vaccine by 2015 that has a protective efficacy of more than 50% against severe disease and death and lasts longer than one year.
- RTS,S vaccine is farthest in the clinical development. The trial has recruited 16000 children belonging to 2 groups (6-14 weeks and 5-17 months). The elder age group showed a decrease in severe malaria in 50% case a/c/t 35 % in younger age (age-dependent differential immune response and lower anti-circumsporozoite antibody titers observed in infants as compared with titers in older children)
- P.knowlesi: Increasing in prevalence. Short erythrocytic cycle of 24 hours. So even if it is easily treatable with chloroquine increased mortality is seen due to late detection.
- Pharmacogenetics
- Amodiaquine: CYP2C8 poor metabolizers a/w increased toxicity.
- Artemisinins: CYP2A6 poor metabolizers. Decreased generation of DHA the active metabolite l/t decreased efficacy.
- WHO: change in guidelines 2010-11:
- Diagnosis to be confirmed before t/t
- Uncomplicated p.f: ACT. Add 0.75 mg/kg primaquine at the end.
- Chloroquine resistance P.vivax: ACT with primaquine for 14 days.
- SEQUAMAT trial and AQUAMAT trials showed that i.v artesuate is better than iv. Quinine for treatment of complicated malaria. With this background WHO in 2011 recommended intravenous artesunate should be used in preference to quinine for the treatment of severe P. falciparum malaria in adults and children. Following this, a full course of an effective should be administered for 7 days.
- National drug policy for malaria-2013
- Ban on artemisinin monotherapy & presumptive chloroquine treatment
- Mixed infection: Treat as falciparum but tail of primaquine recommended
