Placebo
- Latin word – Placere meaning ‘I shall please’
- Hooper’s Medical Dictionary (1811) defined placebo as “any medicine adapted more to please than benefit the patient”
- An intervention designed to simulate a behavioral intervention but not believed (by the investigator) to be a specific therapy for the target condition
- Placebo reactors: Individuals who easily respond to a placebo
- Placebo Response: A positive effect or cure produced by a placebo drug or treatment
- The effect is not caused by the placebo, and is solely due to the person’s belief in treatment and their expectations
Where are placebos used?
- To treat a patient who, in the opinion of the physician, does not require an active drug
- As a control device in clinical trial of drugs
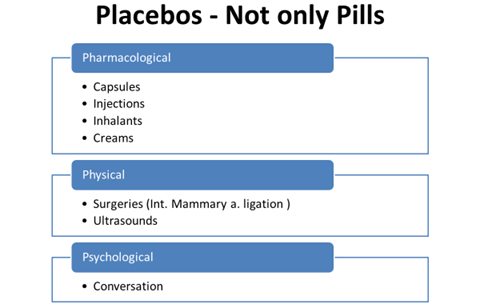
TYPES OF PLACEBO
- Inert or Pure
Substances that have no conceivable pharmacologic effect on the patient
• Dummy pills or capsules containing lactose or chalk
- Active or Impure
Potential pharmacological effects, though not necessarily any specific activity for the condition under treatment
• Antibiotics to treat viral infections
• Vitamin B12 or Fe in absence of anemia
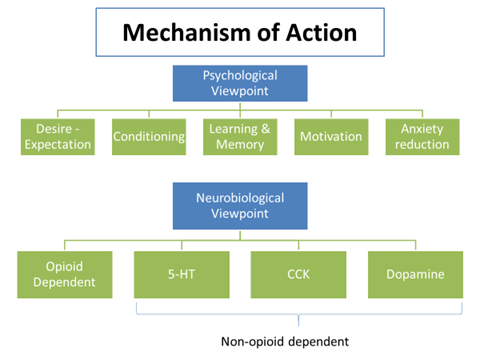
- Placebo effects are accompanied by reduction in neural activity within brain areas that process symptoms of anxiety & pain
- MRI: Prince et al, 2007 found placebo analgesia related to decreased neural activity in pain processing areas of brain thalamus, anterior insular cortex & anterior cingulate cortex during placebo treatment compared to baseline condition
Evidence for involvement of opioids
- Placebo may act by triggering the release of endogenous opioids (endorphins and enkephalins) in CNS.
Placebo effects can be completely or partly reversed by the opioid antagonist naloxone
Placebo in Clinical Trials:
- A placebo-controlled trial is one in which treatment with a placebo is compared with treatment with a test drug.
- In a placebo-controlled trial, subjects are assigned, almost always by randomization, to either a test treatment or to a placebo.
- A placebo formulation must match the active drug formulation in
- Shape
- Size
- Color
- Weight
- Smell
why do we use placebo in clinical trials’?
- Ability to Minimize Bias
- The placebo-controlled trial, using randomization and blinding, generally minimizes subject and investigator bias.
- Measures Absolute Efficacy and Safety
- The placebo-controlled trial measures the total pharmacologically mediated effect of treatment. In contrast, an active control trial or a dose-comparison trial measures the effect relative to another treatment.
- The placebo-controlled trial also allows a distinction between adverse events due to the drug and those due to the underlying disease.
- Minimizing the Effect of Subject and Investigator Expectations
- Use of a blinded placebo control may decrease the amount of improvement resulting from subject or investigator expectations because both are aware that some subjects will receive no active drug. This may increase the ability of the study to detect true drug effects.
- Controls for the psychological aspects of being in a clinical trial.
- Helps control for adverse events being attributed to a medicine when they result from spontaneous changes in the disease or from other causes.
- Enables randomization & blinding procedures to be used.
- Double Dummy technique – Drug A + Placebo B vs. Drug B + Placebo A
- Efficient – detect treatment effects with a smaller sample size than any other type of concurrently controlled study
Disadvantages of Placebo-controlled Trials
- Ethical concerns
When a new treatment is tested for a condition for which no effective treatment is known, there is usually no ethical problem with a study comparing the new treatment to placebo
Use of a placebo control may raise problems of ethics, acceptability, and feasibility, when an effective treatment is available for the condition under study in a proposed trial
- Declaration of Helsinki: “In any medical study, every patient–including those of a control group, if any- should be assured of the best proven diagnostic and therapeutic method”
- USA sponsored experiments in developing countries that test various methods of reducing the vertical transmission of HIV
- The trials were strongly criticized as unethical because women received a placebo despite the fact that zidovudine had already been clearly shown to cut the rate of vertical transmission and was given routinely in many developed countries
b. Patient and physician practical concerns
c. Generalizability
d. No comparative information
When to use Placebo-control
- There is no standard treatment
- Standard treatment has been shown to be no better than placebo
- Evidence causes doubt about therapeutic advantage of standard therapy
- Standard therapy has toxicity so severe that many patients have refused to receive it
Investigating the Placebo effect
Novel study designs are needed for the placebo response like:
- The balanced placebo design (BPD)
- Half of the study sample receives placebo and the other half the drug
- This allows to differentiate between the “true” drug response and the “true” placebo response
- Open- Hidden paradigm
- In this paradigm, the patient can receive a treatment in the standard clinical “open” manner, where the treatment is given by the clinician and in full view of the patient. Alternatively, the treatment can be received in a “hidden” manner, by means of a computer-programmed drug infusion pump, where the clinician is not present and the patient is unaware that the treatment is being administered
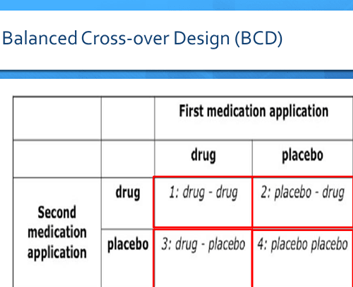
- The balanced cross-over design (BCD)
- Subjects are divided into four groups, and all are told they participate in a conventional randomized double-blinded and placebo-controlled crossover trial
- However, only group 2 & 3 will be exposed to drug and placebo in a balanced way
- Group 1 will receive drug – drug, and Group 4 will receive placebo – placebo instead
- In Group 1, the minimal value of both measures represents the “true” drug response, and the difference between both is the expectancy component of the drug response
- In Group 4, the maximum value should represent the “true” placebo response; and the difference between both values should be the expectancy component of the placebo response
Why adverse effects with placebo ?
- Adverse reaction was listed in the consent form.
- It may be characteristic of a class of drugs which was experienced by the patient before.
- Contact reaction – proximity to others who might get the reaction may influence.
- The patient may learn about it through literature on the drugs.
- It may be a part of the disease itself.
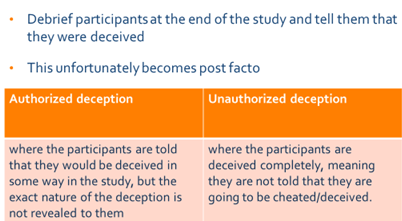
Deception
Scientific research aims to discover and communicate the truth. However, deceptive techniques hold the truth back. The very use of deception precludes the participant from making an informed decision. In addition, it may manipulate individuals to volunteer when they otherwise would not have chosen to do so had they been informed accurately about the nature of the research, including its use of deception.
deception is said to occur when investigators intentionally communicate in a way that produces false beliefs. Investigators may deceive subjects by intentionally giving them false information or by intentionally with-holding information in order to produce false beliefs
Nocebo
- Coined by Walter Kennedy in 1961
- Latin word ‘nocebo’ means “I shall harm”
- Inert substance that creates harmful effects on the patient
- “Nocebos,” or negative placebos, can also be quite powerful. Researchers at UCLA told psychology students who had volunteered for a study that a mild electric current would be passed through their skulls, and that they might experience a headache. Although there was in fact no such current, more than two-thirds of the students reported that they felt pain “
