Probiotics
Definition- ‘‘Live microorganisms which when administered in adequate amounts confer a health benefit on the host’’ (WHO)
- Prebiotic – a substance (usually an oligosaccharide) that cannot be digested but does promote the growth of beneficial bacteria or probiotics
- E.g. Oligofructose, Inulin, Galacto-oligosaccharides, Lactulose, Breast milk oligosaccharides
- Symbiotic – a substance containing both a prebiotic and probiotic.
- Ellie Metchnikoff, the first scientist who proposed the therapeutic use of lactic acid bacteria.
Why are Probiotics important for Human health?
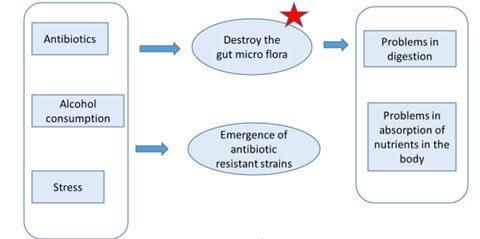
Key properties of probiotic
- Non-pathogenic, non-toxic and non-allergic.
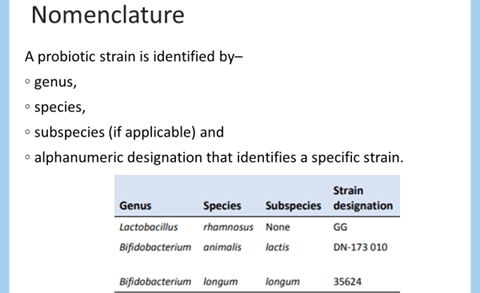
- The organism must be fully identified: genus, species and strain
- Capable of surviving and metabolizing in upper G.I. tract e.g. Resistant to low pH, organic acids, bile juice, saliva and gastric acid
- Human in origin, genetically stable and capable of remaining viable for long periods in field condition.
- Able to modulate immune response and provide resistance to disease through improved immunity or by the production of antimicrobial substance in the guts.
- Good adhesion/ colonization to human intestinal tract and influence on gut mucosal permeability.
- Antagonistic against pathogenic organisms.
- Clinically proven health benefit, e.g. gastrointestinal disorders, diarrhoea, clostridium difficle colitis, antibiotics associated diarrhoea, acute gastroenteritis.
- Technologic properties for commercial viability such as stability of desired characteristics during processing, storage and transportation.
Examples of probiotics
Lactobacillus species –L. acidophilus, L. casei (rhamnosus), L. johnsonii, L. bulgaricus, L. plantarum, L. lactis
Bifidobacterium species– B. bifidum, B. longum, B. infantis, B. lactis, B. adolescentis.
Others- Bacillus cereus, Non-pathogenic Escherichia coli, Saccharomyces cerevisiae (yeast), Enterococcus faecalis
Streptococcus thermophilus.
FMT (Faecal Microbiota Transplant) cannot be considered probiotic, because this involves an undefined mixture of micro-organisms.
Mechanism of action
- Bioconversion , for example, sugars into fermentation products
- Production of growth substrates, like vitamins B and K, for other bacteria
- Direct antagonism to antimicrobial substances:
- hydrogen peroxide
- organic acids
- Bacteriocin
- Acidophilin
- Improve absorption of nutrients
- Production of β- D- galactosidase enzymes that break down lactose .
- Reduction of inflammation, thus altering intestinal properties
- Stimulation of innate immune response
Foods containing Probiotics
- Milk, Soya milk, Milk products– Sour cream, Butter milk, Yoghurt, Fermented Indian foods like Idlis, Dosas, uttapam, Dhoklas, Vadas, Kadhi.
Genetically Engineered Probiotics
- A genetically modified Bacteroides ovatus and Xylan in small amounts as a therapy to animals with colitis.
- ViBact (which is made up of genetically modified Bacillus mesentricus), which acts as an alternate to B-complex capsules.
Benefits of Genetic engineering:
- Strengthen the effects of existing strains
- As vector for vaccines and growth hormones
Clinical application of probiotics
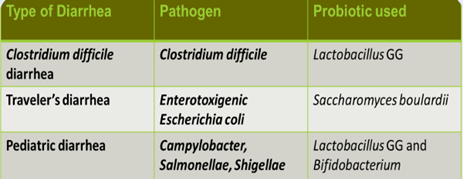
results are promising but not yet conclusive.
Diarrheal Illness – treatment and prevention
- Prevention of antibiotic-associated diarrhea (AAD)
- Genito-urinary.
- Inflammatory bowel disease (ulcerative colitis,CD)
- Lactose intolerance
- Cancers
- Food allergies
- Eczema
- Necrotizing enterocolitis
Allergy
- Atopic eczema, Dermatitis, Allergic rhinitis, Asthma, food allergies with peanuts, tree nuts, soy, wheat gluten, fish
- Atopic children tend to have a degree of dysbiosis with more clostridia and fewer bifidobacteria
- é IgE thought to be caused by a skewed balance between T helper type 1 and T helper type 2 cells
- Probiotics improving the Th1/Th2 balance in favor of a predominance of Th1 cells
Genitourinary
- Recurrent Candida Vaginitis and Bacterial Vaginosis have been successfully treated by administration of both oral and vaginal Lactobacilli
- Vaginal pessaries with 1 billion colony forming units
IBD
- IBD is associated with a breakdown of the normal barrier function provided by the gut epithelial lining and its associated with decreased mucus commensal bacteria.
- Use of probiotics and prebiotics giving good results in maintaining remission in UC but not of much in CD
- Nissle strain – Only probiotic recommended in ECCO guidelines as effective alternative to mesalazine in maintenance of remission in UC patients.
- Good evidence for preventing an initial attack of pouchitis, and relapse of pouchitis after the induction of remission with antibiotics
- Can be recommended in mild pouchitis, or as maintenance therapy for those in remission
- Usually 1×109, or 1 billion colony forming units (CFU) is a good daily dose
IBS
- Prebiotics relieve constipating symptoms-
- Increased bacterial mass and osmotic water-binding capacity making stools bulky
- Increased stool frequency and make stools softer
- A reduction in abdominal bloating and flatulence as a result of probiotic treatments is a consistent finding
- Some strains may ameliorate pain and provide global relief (B. infantis 35624)
- Dose – 108 cfu of the freeze-dried probiotic bacteria B. infantis spec.
Immunoregulation
- Probiotics helps to Increase IgA production by B- cells
- Has antiviral property, induce NO synthase (a virus infected cell death mechanism)
- Production of gamma intereferon, TNF-alph,IL-1 by mononuclear cells incubated with Lactobacillus. Increase cytotoxic potential of NK cells, increasing IL-10 synthesis thus preventing acute infectious disease
- Adherant Lactobacilli and Bifidobacteria significantly increase phagacytosis.
Anticancer effects
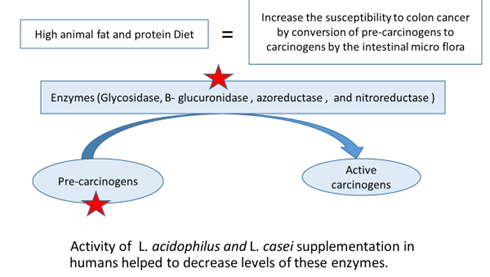
- Probiotics have been shown to improve biomarkers associated with colorectal cancer
- 5-wk supplementation of probiotics reduced the urinary excretion of aflatoxin, a marker for hepatocyte carcinogenesis, and symbiotic consumption for 12 wk significantly reduced colorectal cancer risk
Necrotizing Enterocolitis
- Reduces the risk of necrotizing enterocolitis in preterm neonates.
- Meta-analyses have also shown a reduced risk of death in probiotic-treated groups, although not all probiotic preparations tested are effective.
- NNT to prevent one death from all causes by treatment with probiotics is 20.
Adverse effects
- Probiotics side effects, if they occur, tend to be mild and digestive symptoms. (such as gas or bloating).
- May cause infections, especially in immuno- compromised patients.
- Diabetic patients should be cautious about taking probiotic drinks available in the market as they contain high level of sugar.
Probiotic products taken as a dietary supplement are manufactured and regulated as functional foods, not drugs.
- Probiotics supplements are not necessary for normal, healthy individuals.
- In healthy individuals, daily consumption of probiotics rich foods, particularly fermented dairy products like yoghurt adequately supplies the amount of probiotics required to maintain a healthy digestive system and overall wellbeing.
- A diet high in starches and fibre provide prebiotics, which keep a healthy population of probiotics in our intestines.
Challenges
- Retention of viability of the probiotic bacteria – The numbers of viable bacteria continually decrease with time during refrigerated storage
- A serious problem of shelf instability had been encountered with dried Cultures.
- Manufacturer needs to incorporate an excess of cells at the time the tablets are manufactured. This practice increases the cost and makes the use instructions inaccurate
- Extreme temperatures, high pressure, shear forces can destroy the preperations
- Survival of most bifidobacteria in most dairy products is poor due to low pH and/or exposure to oxygen
- Difficulty in assessment of efficacy of probiotics
- Currently in India, probiotics are characterized as functional food
- Regulated by food laws – FSSA (eatables regulations covering nutraceuticals, foods and dietary supplements)
- ICMR and DBT have formulated guidelines for probiotics being marketed as foods.
- These guidelines specify the criteria when launching a probiotic food, labelling requirements
- They describe parameters to define a product as ‘probiotic’.
- May be used as a reference to establish probiotic standards in the country by the FSSAI
- Probiotics delivered in food MAY NOT be tested in PHASE 3 studies, unless the product makes a specific health claim wherein it becomes necesssary to carry out Phase 3 studies.
