Vancomycin and Other Glycopeptides
- Glycopeptide antibiotics are actinomycete-derived antibiotics with unique tricyclic or tetracyclic heptapeptide cores that are usually glycosylated.
- bactericidal
- inhibits cell wall synthesis by inhibiting transglycosylase enzyme (involved in chain elongation).
- It has narrow spectrum
- effective against gram positive organisms including MRSA, penicillin resistant pneumococci and Clostridium difficile.
- drug of choice for MRSA, Corynebacterium jeikeium and for serious infections in penicillin allergic patients.
- Glycopeptides – most prevalent class of therapeutics against severe infections caused by :
- Gram-positive pathogens:
- 1) Enterococci
- 2) Methicillin-resistant staphylococcus aureus (MRSA)
- 3) Clostridium difficile
- MRSA – Endemic in India à Hospital acquired infection
- A study showed overall prevalence of methicillin resistance as 41 %. Isolation rates for MRSA from OPD, IPD and ICU were 27, 49 and 47 per cent in 2009.
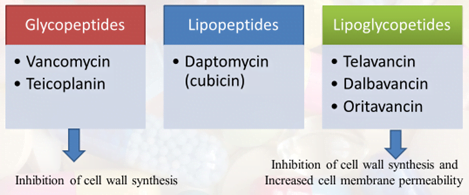
- Classification of glycopeptide antibiotics
Mechanism of action
- The bacterial cell wall- peptidoglycan– structural support.
- Peptidoglycan monomers- sugar backbone with peptide and disaccharide units attached by glycosidic bonds into long chains via Transglycosidation.
- The glycopeptide antibiotics- cell membrane- noncovalent bonds with terminal carbohydrates- inhibition of cross-linking by the Transpeptidase.
- Subsequently, the weakened cell wall- cell cytolysis and death.
Vancomycin
- Primary target: D-Ala-D-Ala terminus of pentapeptidic precursors
- Vancomycin forms complex with the D-Ala-D-Ala residues by forming five hydrogen bonds with the peptide backbone of the glycopeptide.
- This complex prevents the transpeptidation reactions via steric hindrance.
- These are administered parenterally (vancomycin by i.v. route and teicoplanin by i.v. or i.m. route) and are excreted unchanged in urine.
Clinical uses
- Blood stream infections and endocarditis caused by MRSA.
- Enterococcal endocarditis in a patient with serious penicillin allergy (with gentamicin) .
- Meningitis suspected or known to be caused by a penicillin-resistant strain of pneumococcus (with cefotaxime, ceftriaxone, or rifampin)
- Other infections due to staphylococci – septicemias, LRTI, bone, skin and skin structure infections
- Oral vancomycin, 0.125–0.25 g every 6 hours – antibiotic associated pseudomembranous colitis by C. Difficile – because it is not absorbed from the gastrointestinal tract and higher concentration reaches the colon.
Adverse reactions
- Adverse reactions in about 10% of cases (most reactions are minor).
- “Red man” or “red neck” syndrome à Rapid i.v. infusion of high doses of vancomycin can cause RED MAN SYNDROME (diffuse flushing due to histamine release). It is the most common adverse
reaction to vancomycin.
- Phlebitis at the site of injection.
- Chills and fever
- Ototoxicity( rare)and nephrotoxicity uncommon with current preparations.
- Its dose should be decreased in renal failure.
Mechanism of resistance
- Enterococci- mediated by acquirement of a gene à codes for enzymes: synthesis of low- affinity and removal of high- affinity peptidoglycan precursors
- Nine types of vancomycin resistance (Van A to Van N)
- Van A enterococci- resistance induced by Vancomycin and Teicoplanin
- Van B enterococci – sensitive to Vancomycin, resistant to Teicoplanin.
Teicoplanin
- Teicoplanin is another glycopeptide with similar characteristics but can be given once daily due to long t1/2 (45-70 hours).
- The fatty-acid component increased lipophilicityà greater cellular and tissue penetration
- Mechanism
- It inhibits the synthesis of peptidoglycans in the bacterial cell wall by the nonspecific binding
- 2) The saturation of the outer layers of bacterial peptidoglycans.
- 3) Teicoplanin then binds to the D-Ala-D-Ala terminus of the precursors, which fits into a cleft in the teicoplanin molecule
- Teicoplanin does not cause red man syndrome or nephrotoxicity.
- Indicated for treatment of serious infections by staphylococcus or streptococcus
1) Bone- osteomyelitis
2) joints- septic arthritis
3) blood- non cardiac bacteremia, septicemia
- Unlike vancomycin, it can be given IM and IV
- Teicoplanin has a long half-life (45–70 hours), permitting once-daily dosing .
Semisynthetic glycopeptides
Telavancin, Oritavancin and Dalbavancin
Advantages:
1) Overcome the emergence of MRSA strains showing weaken sensitivity to Vancomycin
2) To increase the penetration into tissues and into CSF
3) Longer half-life in comparison with Vancomycin
4) Improvements for infrequent dosing
5) Greater potency
6) Lower risk of development of resistant microorganisms.
Telavancin
- FDA approval in 2009
- A derivative of vancomycin:
- Lipophilic group: increased membrane interactions
- Phosphonate group: improved adsorption, distribution, metabolism and the excretion profile of telavancin.
- Dual mechanism of action– Inhibition of peptidoglycan biosynthesis and membrane depolarization.
- Apart from vancomycin like mechanism, it also disrupts membrane potential.
- approved à treatment of complicated skin and soft tissue infections à 10 mg/kg IV daily.
- Clinical use subsequently extended to include hospital-acquired and ventilator-associated bacterial pneumonia
- potentially teratogenic, so administration to pregnant women must be avoided
- The half-life of telavancin is approximately 8 hours, which supports once-daily intravenous dosing.
Dalbavancin
- Approved in May 2014.
- A semisynthetic derivative of the teicoplanin-like glycopeptide A40926 complex, derived from Nonomuraea sp.
- Three phase-III trials successfully completed between 2003 and 2005, but the FDA required additional non-inferiority data in 2007
- In 2009, two additional phase-III trials were initiated which met their primary endpoint of non-inferiority.
- Amidation of the C-terminal carboxyl group with a dimethylaminopropylamine group produced dalbavancin
- These modifications led to an extended half-life of over 300 h in human allowing for once weekly dosing.
Uses
- Approved for the treatment of adult patients with complicated skin and skin structure infections, including those caused by MRSA
- It is not active against most strains of vancomycin-resistant enterococci.
- Dalbavancin has an extremely long half-life of 6–11 days, which allows for once weekly intravenous administration
Oritavancin
- Approved in August 2014
- Two phase-III trials completed with results disclosed in 2001 and 2003, but the FDA rejected a new drug application (NDA) in 2008 for concerns over safety and effectiveness.
- In 2009, 2 more phase-III trials for Gram-positive ABSSSI completed.
Use
- The injection form is approved for treatment of acute bacterial skin and skin structure infections (ABSSSI)
- Dimer formation- residual activity against vancomycin-resistant bacteria.
- Intrinsic bactericidal activity especially against streptococci
- Effectiveness – not affected by the antibiotic-resistance mechanisms developed by staphylococci and enterococci, effective in VRSA and VRE
Spectrum of activity
- Aerobic gram-positive microorganisms
- Listeria monocytogenes
- Streptococcus pyogenes
- Streptococcus pneumoniae (including penicillin-resistant strains)
- Streptococcus agalactiae
- Anaerobic gram-positive microorganisms
- Actinomyces species
- Lactobacillus species
Drugs under trial

