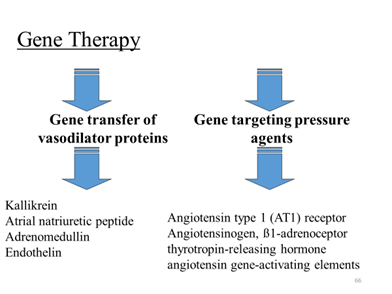
CARDIAC GLYCOSIDES
- having cardiac inotropic property.
- increase myocardial contractility and output in a hypodynamic heart without a proportionate increase in O2 consumption. Thus, efficiency of failing heart is increased.
- In contrast, ‘cardiac stimulants’ (Adr, theophylline) increase O2 consumption rather disproportionately and tend to decrease myocardial efficiency.
- William Withering àdigitalis
Heart
- Digitalis has direct effects on myocardial contractility à dose dependant
- In addition, it has vagomimetic action. à Vagal tone is increased reflexly by sensitization of baroreceptors, as well as by stimulation of vagal centre.
- Heart rate is decreased by digitalis à Bradycardia
- it generates increased tension so that stroke volume is maintained upto considerably higher values of impedance
- The digitalized failing heart regains some of its capacity to contract more forcefully when subjected to increased resistance to ejection.
- cardiac output is increased and end-diastolic volume is reduce
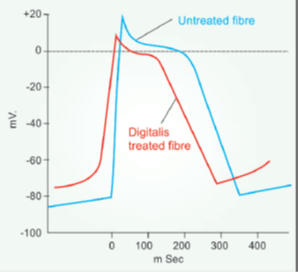
- The resting membrane potential (RMP) is progressively decreased with increasing doses.
- The rate of 0 phase depolarization is reduced resulting in slowing of conduction.
- slope of phase-4 depolarization is increased in the PFs
- action potential duration (APD) is reduced (primarily at phase-2) and amplitude of AP is diminished.
- A-V conduction is demonstrably slowed by therapeutic doses.
Mechanism of action
- electively binds to extracellular face of the membrane associated Na+K+ATPase of myocardial fibres and inhibits this enzyme
- progressive accumulation of Na+ intracellularly. This indirectly results in intracellular Ca2+ accumulation.
- triggers release of larger amount of Ca2+ stored in sarcoplasmic reticulum (SR) through Ryanodine calcium channel 2 (RYR2) à cytosolic Ca2+ increases transiently.
- The relationship of cytosolic [Na+] and [Ca2+] is such that a small percentage increase in Na+ concentration leads to a large percentage increase in Ca2+ concentration.
- At high doses, there is depletion of intracellular K+; and digitalis toxicity is partially reversed by infusing K+, because K+ decreases binding of glycoside to Na+K+ ATPase.
- HTN and coronary insufficiency à not a C/I for Digitalis use
- Diuresis occurs promptly in CHF patients, secondary to improvement in circulation and renal perfusion. No diuresis occurs in normal individuals
- Presence of food in stomach delays absorption of digoxin
- Digoxin is the only cardiac glycoside available widely and used clinically now.
ADVERSE EFFECTS
- margin of safety is low (therapeutic index 1.5–3).
- Higher cardiac mortality has been reported among patients with steady-state plasma digoxin levels > 1.1 ng/ml but still within the therapeutic range during maintenance therapy.
- Anorexia, nausea, vomiting and abdominal pain are usually reported first: are due to gastric irritation, mesenteric vasoconstriction and CTZ stimulation
- Almost every type of arrhythmia can be produced by digitalis
- Partial to complete A-V block may be the sole cardiac toxicity
Treatment
- Further doses of digoxins must be stopped at the earliest sign of toxicity; nothing more needs to be done in many patients, especially if the manifestations are only extracardiac.
- Tachyarrhythmias à caused by chronic use of digitalis and diuretics (both induce K+ depletion)—infuse KCl 20 m.mol/hour (max. 100 m. mol) i.v.
- For ventricular arrhythmias à Lidocaine i.v.
- For supraventricular arrhythmias à Propranolol i.v. or orally
- For A-V block and bradycardia àAtropine 0.6–1.2 mg i.m. may help; otherwise cardiac pacing is recommended.
- Digoxin specific antibody à DIGIBIND (38 mg vial) à nonimmunogenic because it lacks the Fc fragment. Given by i.v. infusion à The digoxin-Fab complex is rapidly excreted by kidney
- (a) Hypokalemia: enhances digitalis toxicity.
- (b) Elderly, renal or severe hepatic disease: patients are more susceptible to digoxin toxicity
- Wolff-Parkinson-White syndrome à Digitalis is contraindicated because it decreases the ERP of bypass tract in 1/3 patients.
INTERACTIONS
- 1. Diuretics: cause hypokalemia which increases the risk of digitalis arrhythmias; potassium supplements should be given prophylactically.
- 2. Calcium: synergises with digitalis → precipitates toxicity.
USES
- The two main indications of digitalis are CHF and control of ventricular rate in atrial fibrillation/flutter
1. Congestive heart failure
- Heart failure may primarily be due to systolic dysfunction or diastolic dysfunction.
Systolic dysfunction
- The ventricles are dilated and unable to develop sufficient wall tension to eject adequate quantity of blood.
- This occurs in ischaemic heart disease, valvular incompetence, dilated cardiomyopathy, myocarditis, tachyarrhythmias (mostly atrial fibrillation).
Diastolic dysfunction
- The ventricular wall is thickened and unable to relax properly during diastole; ventricular filling is impaired because of which output is low.
- occurs in sustained hypertension, aortic stenosis, congenital heart disease, A-V shunts, hypertrophic cardiomyopathy.
- However, most patients, especially longstanding CHF, have both systolic and diastolic dysfunction.
- Cardiac glycosides afford only symptomatic relief, primarily in systolic dysfunction.
- Best results are obtained when myocardium is not primarily deranged, e.g. in hypertension, valvular defects or CHF due to rapid heart rate in atrial fibrillation.
- Poor response and more toxicity is likely when the myocardium has been damaged by ischaemia, inflammation or degenerative changes and in thiamine deficiency, as well as in high output failure (in anaemia).
- Digitalis induced enhancement of contractility increases ventricular ejection
- Improved tissue perfusion results in withdrawal of sympathetic overactivity à heart rate and central venous pressure (CVP) are lowered towards normal.
- Liver regresses, pulmonary congestion is reducedà dyspnoea abates, cyanosis disappears. Low output symptoms like decreased capacity for muscular work are mitigated.
- Wall tension = Intraventricular pressure × ventricular radius
- i.e. to generate the same ejection pressure a dilated ventricle has to develop higher wall tension. à By reducing end diastolic volume (due to better emptying), digitalis restores efficiency of translation of cardiac work into cardiac output. That is why O2 consumption does not increase proportionately.
- Current status
- Now ACE inhibitors/ ARBs, β adrenergic blockers and diuretics are the standared treatment.
- However, digitalis is still the most effective drug capable of relieving symptoms of CHF and restoring cardiac compensation, especially in patients with dilated heart and low ejection fraction.
- All patients who remain symptomatic even while receiving ACE inhibitor/ARB, β blocker and diuretic should be treated with digitalis.
2. Cardiac arrhythmias
- Atrial fibrillation (AF)
- Digitalis is used for controlling ventricular rate in AF, whether associated with CHF or not.
- However, it is incapable of curing AF
- Digitalis reduces ventricular rate in AF by decreasing the number of impulses that are able to pass down the A-V node and bundle of His
- particularly effective in controlling ventricular rate at rest, but has less effect during exercise. Thus, it is preferred in sedentary patients.
- For physically active patients, ß blockers/verapamil/diltiazem provide better rate control.
- Atrial flutter (AFI) The atrial rate is 200–350/ min (less than that in AF)
- Digitalis may convert AFl to AF by reducing atrial ERP and making it inhomogeneous. This is a welcome response because control of ventricular rate is easier in AF
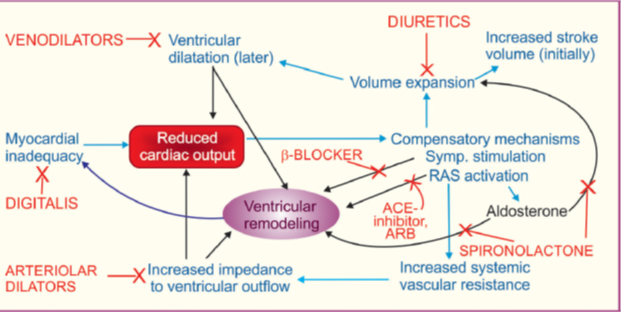
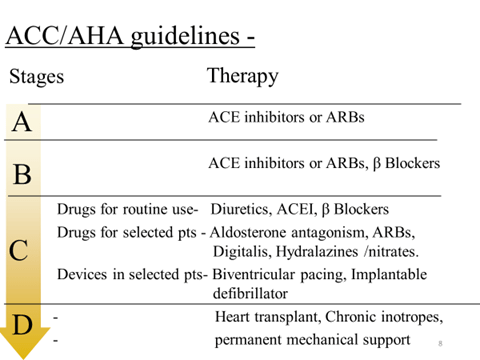
Drugs for Heart Failure
There are two distinct goals of drug therapy in CHF:
- Relief of congestive/low output symptoms and restoration of cardiac performance.
This can be achieved by:
- Inotropic drugs—Digoxin, dobutamine/ dopamine, amrinone/milrinone
- Diuretics—Furosemide, thiazides
- RAS inhibitors—ACE inhibitors/ARBs
- Vasodilators—hydralazine, nitrate, nitroprusside
- Synthetic BNP à Nesiritide
- β blocker—Metoprolol, bisoprolol, carvedilol, Nebivolol
(b) Arrest/reversal of disease progression and prolongation of survival, possible with:
- ACE inhibitors/ ARBs, β blockers
- Aldosterone antagonist—Spironolactone, eplerenone
- Neprilysin inhibitor à Sacubitril
nonpharmacological measures
- Rest and salt restriction.
- Salt restriction limits edema formation and is advised in all grades of CHF.
Diuretics
- Almost all cases of symptomatic CHF are treated with a diuretic.
- High ceiling diuretics (furosemide, bumetanide) are the diuretics of choice for mobilizing edema fluid; later they may be continued in low doses.
- In advanced CHF after chronic use, resistance may develop to even high ceiling diuretics.
- Addition of a thiazide/ metolazone/spironolactone to furosemide may overcome the resistance.
Diuretics:
(a) Decrease preload and improve ventricular efficiency by reducing circulating volume.
(b) Remove peripheral edema and pulmonary congestion.
- Intravenous furosemide promptly increases systemic venous capacitance and produces rapid symptomatic relief in acute left ventricular failure.
- they may cause activation of RAS (if hypovolemia occurs) which has adverse cardiovascular consequences.
- Chronic diuretic therapy tends to cause hypokalemia, alkalosis and carbohydrate intolerance.
- Current opinion is to treat mild heart failure with ACE inhibitors/ARBs ± β blockers only, because they afford survival benefit, while diuretics may be added intermittently for symptom relief.
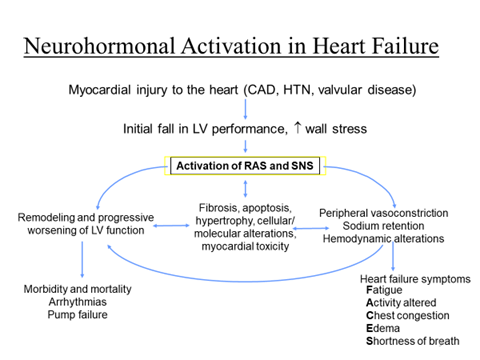
Renin-angiotensin system (RAS) inhibitors
- Since RAS activation is pivotal to development of symptoms and disease progression in CHF
- ACE inhibitors and ARBs à afford symptomatic as well as disease modifying benefits in CHF by causing
- vasodilatation,
- retarding/preventing ventricular hypertrophy,
- myocardial cell apoptosis,
- fibrosis intercellular matrix changes and remodeling.
- In addition to decreasing Ang II production, ACE inhibitors raise the level of kinins which stimulate generation of cardioprotective NO and PGs.
- Symptomatic and prognostic benefits à mild to severe (NYHA class I to IV) CHF as well as in subjects with asymptomatic systolic dysfunction.
- thus recommended for all grades of CHF, unless contraindicated, or if renal function
Vasodilators
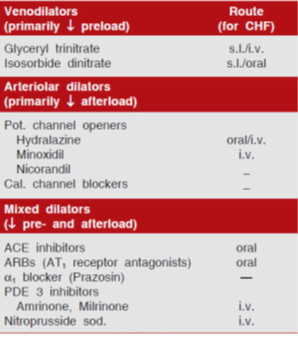
- Vasodilators à i.v. to treat acute heart failure that occurs in advanced cases or following MI, and serve to tide over crisis.
- oral route à long-term therapy of chronic CHF, but vasodilators other than ACE inhibitors/ARBs have only limited utility.
(i) Preload reduction:
- Nitrates cause pooling of blood in systemic capacitance vessels to reduce ventricular end-diastolic pressure and volume.
- With reduction in size of ventricles, effectiveness of myocardial fibre shortening in causing ejection of blood during systole improves (Laplace relationship).
- Controlled i.v. infusion of glyceryl trinitrate affords rapid relief in acute left ventricular failure, particularly that due to myocardial ischaemia/infarction.
- However, lowering of preload (by vasodilators + strong diuretics) beyond a limit may reduce output of a failing heart whose performance is dependent upon elevated filling pressure.
- Occurrence of nitrate tolerance limits their utility in routine treatment of CHF.
(ii) Afterload reduction
Hydralazine
- Dilates resistance vessels and reduces aortic impedance so that even weaker ventricular contraction is able to pump more blood; systolic wall stress is reduced.
- It is effective in forward failure when cardiac index (CI = min output/body surface area) is low (< 2.5 L/min/m2) without a marked increase in CVP (< 18 mm Hg).
- Marked tachycardia, worsening of myocardial ischaemia and fluid retention limit long-term use of hydralazine monotherapy.
- Minoxidil
- more potent arteriolar dilator, but has found little use in heart failure; so has nicorandil a more
- specific pot. channel opener.
- Trials of the three prototype calcium channel blockers verapamil, diltiazem and nifedipine in systolic dysfunction have been disappointing, even negative with occasional worsening of symptoms and increase in mortality.
- This may be due to reflex sympathetic activation (nifedipine) or negative inotropic property (verapamil,
- diltiazem).
- Verapamil, however, is useful in diastolic dysfunction due to hypertrophic cardiomyopathy.
- Trials with long-acting and more vasoselective dihydropyridines (felodipine, amlodipine) have also not been encouraging.
(iii) Pre- and after load reduction
Sod. nitroprusside
- high efficacy i.v. dilator with equal action on the two types of vessels.
- acts by both the above mechanisms, i.e. reduces ventricular filling pressure as well as systemic vascular resistance.
- Cardiac output and renal blood flow are increased.
- Titrated i.v. infusion of nitroprusside is employed in conjunction with a loop diuretic + i.v. inotropic drug to tide over crisis in severely decompensated patients.
- For symptomatic treatment of acute heart failure, choice of i.v. vasodilator (glyceryl trinitrate or hydralazine or nitroprusside) depends on the primary haemodynamic abnormality in individual patients.
- In the long term oral therapy, survival benefit has been obtained only with a combination of hydralazine + isosorbide dinitrate, but the ACE inhibitors and ARBs are clearly superior in this regard
- Hydralazine alone or a nitrate alone have not proven useful in the treatment of chronic heart failure.
- However, when combined they supplement each other and nitrate tolerance is attenuated by hydralazine.
β-Adrenergic blockers
- β1 blockers (mainly metoprolol, bisoprolol, nebivolol) and the nonselective β + selective α1 blocker carvedilol in mild to moderate CHF treated with ACE inhibitor ± diuretic, digitalis.
- A large number of randomized trials have demonstrated subjective, objective, prognostic and mortality benefits of the above named β blockers over and above that afforded by ACE inhibitors + diuretic ± digitalis.
- Though the immediate hemodynamic action of β blockers is to depress cardiac contractility and ejection fraction, these parameters gradually improve over weeks.
- After a couple of months ejection fraction is generally higher than baseline, and slow upward titration of dose further improves cardiac performance.
- The benefits appear to be due to antagonism of ventricular wall stress enhancing, apoptosis promoting and pathological remodeling effects of excess sympathetic activity (occurring reflexly) in CHF, as well as due to prevention of sinister arrhythmias.
- Incidence of sudden cardiac death as well as that due to worsening CHF is decreased.
- β blockers lower plasma markers of activation of sympathetic, renin-angiotensin systems and endothelin-1.
- Greatest utility of β blockers has been shown in mild to moderate (NYHA class II, III) cases of dilated cardiomyopathy with systolic dysfunction in which they are now routinely co-prescribed unless contraindicated.
- Encouraging results (upto 35% decrease in mortality) have been obtained in class IV cases as well, but use in severe failure could be risky and needs constant monitoring.
- There is no place for β blockers in decompensated patients. β blockers should be stopped during an episode of acute heart failure and recommenced at lower doses followed by uptitration after compensation is restored.
- Starting dose should be very low—then titrated upward as tolerated to the target level (carvedilol 50 mg/day, bisoprolol 10 mg/day, metoprolol 200 mg/day) or near it, for maximum protection.
- long-acting preparation (e.g. sustained release metoprolol) or 2–3 times daily dosing to produce round-the-clock β blockade should be selected.
Aldosterone antagonist (Spironolactone, Eplerenone)
- rise in plasma aldosterone in CHF, in addition to its well known Na+ and water retaining action, is an important contributor to disease progression by direct and indirect effects:
- (a) Expansion of e.c.f. volume → increased cardiac preload.
- (b) Fibroblast proliferation and fibrotic change myocardium → worsening systolic dysfunction and pathological remodeling.
- (c) Hypokalemia and hypomagnesemia → increased risk of ventricular arrhythmias and sudden cardiac death.
- (d) Enhancement of cardiotoxic and remodeling effect of sympathetic overactivity.
- The aldosterone antagonist spironolactone is a weak diuretic, but can benefit CHF by antagonizing the above effects of aldosterone.
- Though ACE inhibitors themselves lower aldosterone levels, this effect is incomplete and short lasting.
Current evidence
- It is indicated as add-on therapy to ACE inhibitors + other drugs in moderate-to-severe CHF.
- It can retard disease progression, reduce episodes of decompensation and death due to heart failure as well as sudden cardiac deaths, over and above the protection afforded by ACE inhibitors/ARBs ± β blockers.
- Only low doses (12.5–25 mg/day) of spironolactone should be used to avoid hyperkalaemia; particularly because of concurrent ACE inhibitor/ARB therapy.
- contraindicated in renal insufficiency because of risk of hyperkalemia—requires serum K+ monitoring.
- Gynaecomastia
Sympathomimetic inotropic drugs
- Drugs with β adrenergic and dopaminergic D1 agonistic actions have positive inotropic and (at low doses) vasodilator properties which may be utilized to combat emergency pump failure.
- Dobutamine (2–8 μg/kg/min) a relatively selective β1 agonist with prominent inotropic action is the preferred drug for i.v. infusion in acute heart failure accompanying myocardial infarction (MI), cardiac surgery as well as to tide over crisis in advanced decompensated CHF.
- Dopamine (3–10 μg/kg/min by i.v. infusion) has been used in cardiogenic shock due to MI and other causes.
- While dobutamine does not raise (may lower) systemic vascular resistance and is preferred in heart failure, dopamine tends to increase afterload, especially at higher rates of infusion (>5 μg/kg/min) and has limited utility in patients who are not in shock.

- This can restore diuretic response to i.v. furosemide in refractory CHF.
- These drugs afford additional haemodynamic support over and above vasodilators, digitalis and diuretics, but benefits are short-lasting.
- no role in the long-term management of CHF.
Phosphodiesterase 3 inhibitors
- Inamrinone (amrinone) à selective (PDE3) inhibitor.
- The PDE3 isoenzyme is specific for intracellular degradation of cAMP in heart, blood vessels and bronchial smooth muscles.
- Amrinone increases myocardial cAMP and transmembrane influx of Ca2+.
- The two most important actions of amrinone are positive inotropy and direct vasodilatation: has been called an ‘inodilator’.
- Both preload and afterload on the heart is reduced.
- Compared to dobutamine, proportionately greater decrease in systemic vascular resistance is noted.
- It increases cardiac index, left ventricular ejection fraction and decreases peripheral vascular resistance, CVP, left ventricular end diastolic volume and pressure accompanied by mild tachycardia and slight fall in BP.
- Adverse effects à Thrombocytopenia à mostly transient and asymptomatic.
Use
- Though amrinone is active orally, its oral use in maintenance therapy of CHF has been abandoned, because efficacy was lost and mortality was increased in comparison to placebo.
- It is indicated only for short-term i.v. use in severe and refractory CHF, as an additional drug to conventional therapy with digitalis, diuretics and vasodilators.
- Milrinone à more selective for PDE3, and is at least 10 times more potent.
- Thrombocytopenia is not significant.
- preferred over amrinone and should be restricted to short-term use only.
Levosimendan – FAILED
- inotropic drug
- acts by sensitizing myocardial troponin C to Ca2+ , as well as by inhibiting PDE3.
- also opens ATP-sensitive K+ channels in vascular smooth muscle cells to cause vasodilatation.
- Thus, it is another ‘ inodilator’
- Haemodynamic improvement in heart failure is both due to increase in cardiac contractility as well as reduction in preload and afterload.
- infused i.v. is primarily indicated for short-term treatment of acutely decompensated severe chronic heart failure.
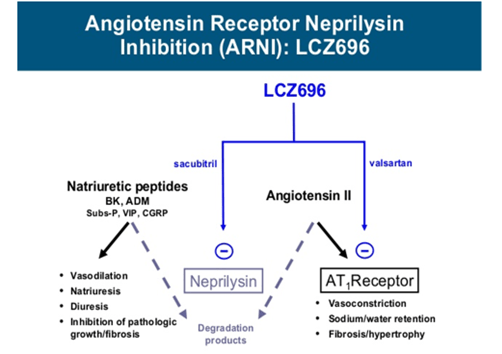
Neutral endopeptidase (NEP; neprilysin) inhibitors:
- new class of drugs which inhibit NEP enzymes that degrade Ps, bradykinin, substance P and adrenomedullin.
- By raising levels of natriuretic and vasodilator peptides they have potential utility in CHF.
- A nonpeptide EP inhibitor Sacubitril has been approved (in 2015) for the treatment of class lI to IV CHF when combined with the ARB valsartan.
- NEP inhibition à increases the blood levels of NPs and other vasodilator peptides resulting in natriuresis, diuresis, vascular relaxation, lowering of Ang ll as well as aldosterone levels
- prodrug à rapidly activated by esterases to sacubilrilat.
- PARADIGM-HF, 2014 trail, a 1:1 combination of sacubitril + valsartan (200 mg BD) was compared with enalapril ( IO mg BD) in 8442 patients of class II to class IV CHF already receiving CHF therapy with one or more of diuretics, beta blockers and aldosterone antagonist.
- The sacubitril-valsartan combination caused 20% greater reduction in death or hospitalization for heart failure than enalapril.
- side effects à hypotension, hyperkalemia and renal impairment.
- sacubitril-valsartan appears to be a more effective and well tolerated alternative to ACE inhibitors for reducing morbidity and mortality in patients with advanced heart failure.
- However, it is expensive.
Nesiritide
- recombinant brain natriuretic peptide (BNP) à approved for i.v. use to relieve dyspnea and other symptoms in refractory CHF.
- enhances salt and water excretion and is a potent vasodilator with profile of action similar to i.v. glyceryl trinitrate; reduces ventricular filling pressure.
- Additional hemodynamic and symptomatic improvement can be obtained for short-periods, but no long term benefits are evident in CHF.
Tolvaptan
- orally active nonpeptide vasopressin V2 receptor antagonist
- for the correction of water retention and hyponatremia occurring in ‘syndrome of inappropriate ADH secretion’ (SIADH) as well as in advanced CHF.
Tolvaptan failed to improve dyspnea despite enhancing early weight reduction. Other clinical end points such as length of stay, incidence of worsening heart failure, or post-discharge outcomes were not reduced
Recent Advances In Management Of Heart Failure
Definition
“Heart failure (HF) is a clinical syndrome characterised by symptoms (dyspnoea, orthopnoea, lower limb swelling) and signs (elevated jugular venous pressure, pulmonary congestion) often caused by a structural and/or functional cardiac abnormality resulting in reduced cardiac output and/or elevated intracardiac pressures”
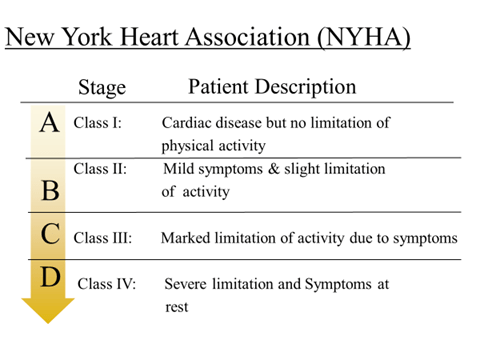
Mortality
- NYHA class II-III: 10-20 % per year
- NYHA class IV: 40-60 % per year
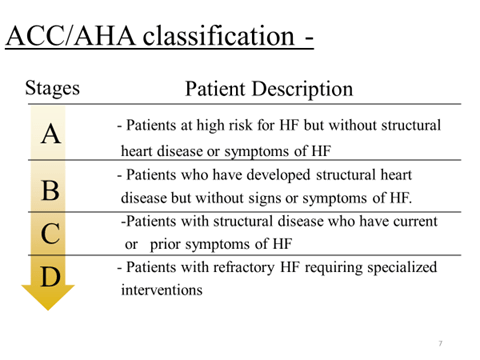
Classification
- BY EJECTION FRACTION
- Reduced ejection fraction (≤ 40 %)- systolic heart failure
- Preserved ejection fraction (≥ 50%)- diastolic heart failure
- BY TIME COURSE
- Chronic heart failure (CHF)
- Acute heart failure
- ANATOMICALLY
- Left sided- LHF
- Right sided- RHF(CHF)
- BY OUTPUT
- High output failure- Thyrotoxicosis, Anaemia, Pregnancy, A-V fistula
- Low output failure – 95% of HF is this
Need for newer drug therapies :
- Available drugs do not control progression of disease effectively
- Associated side effects are more
- Needed life long treatment
- HF is associated with high morbidity and mortality
Newer Drugs –
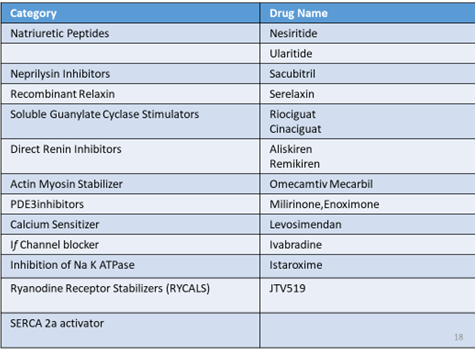
Ivabradine
- Approved in april 2015 for CHF
- MOA : cyclic nucleotide-gated channel blocker that reduces the spontaneous pacemaker activity of the cardiac sinus node by selectively inhibiting the If current (If)
- recommended for patients with stable, symptomatic chronic heart failure with left ventricular ejection fraction (LVEF) 35% or less in sinus rhythm with resting hazard ratio at least 70bpm based on the SHIFT trial
- Indication – stable, symptomatic CHF with LVEF ≤ 35%, and either are on maximally tolerated doses of beta blockers or have a contraindication to beta-blocker use
- Starting dose 5 mg twice daily. After 2 weeks of treatment, the dose adjusted based on heart rate. The maximum dose 7.5 mg BD
- RCT comparing with placebo in 6,558 adults with stable NYHA class II to IV heart failure, left ventricular ejection fraction ≤ 35%, and resting heart rate ≥ 70 bpm
- Result – reduced the risk of hospitalization for worsening heart failure or cardiovascular death
- Adverse effects : bradycardia, hypertension, atrial fibrillation
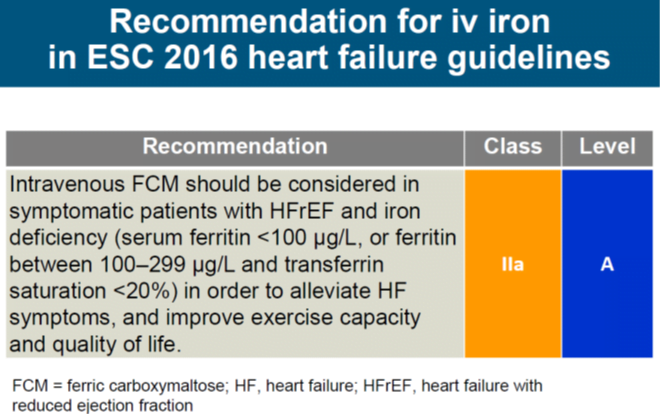
- A meta-analysis published in 2017 reported reduced rates of recurrent hospitalizations in patients with HFrEF and iron deficiency when treated with intravenous iron therapy
- Even though oral iron substitution would be desirable, the available data from several large trials, including the Ferric CarboxymaltOse evaluatioN on perFormance in patients with IRon deficiency in coMbination with chronic Heart Failure, the Ferinject Assessment in Patients with Iron Deficiency and Chronic Heart Failure, the Effect of Ferric Carboxymaltose on Exercise Capacity in Patients With Iron Deficiency and Chronic Heart Failure and the Iron Repletion Effects on Oxygen Uptake in Heart Failure trials, suggest that a positive effect can only be maintained with intravenous iron substitution, preferably in the form of ferric carboxymaltose (CONFIRM-HF trial)
Sacubitril and Valsartan
- Approved July 2015 for chronic heart failure
- MOA – a) sacubitril, a neprilysin inhibitor, inhibits neprilysin via LBQ657 (the active metabolite of the prodrug sacubitril) and
b) valsartan, an angiotensin II receptor blocker
- Indication : patients with chronic heart failure (NYHA Class II-IV) and reduced ejection fraction
- PARADIGM-HF, randomized, double-blind trial comparing Sacubitril and Valsartan with enalapril in 8,442 adult patients with symptomatic CHF (NYHA class II–IV) and systolic dysfunction (LVEF ≤ 40%)
- Starting dose of is 49/51 mg (sacubitril/valsartan) BD
the target maintenance dose of 97/103 mg (sacubitril/valsartan) BD, as
tolerated by the patient after 2 to 4 weeks
- Results – Sacubitril and Valsartan superior to enalapril in reducing the risk of cardiovascular death or hospitalization for heart failure
Recommendation for digoxin use
Current recommendations indicate that digitalis glycosides may be considered for rate control in patients with AF and HFrEF if BB did not provide satisfactory rate control.
Furthermore, digoxin received a IIb recommendation from the ESC in 2016 for use in patients with HFrEF and stable sinus rhythm who were still symptomatic despite optimal medical therapy with ACEI, MRA and BB

Drugs in Pipeline
Patiromer
- MOA – Patiromer binds potassium in exchange for calcium throughout the GIT. Also increases fecal excretion of potassium and consequently decreases plasma potassium levels
- The OPAL-HK trial: The OPAL-HK (A Two-Part, Single-Blind, Study Evaluating the Efficacy and Safety of Patiromer for the Treatment of Hyperkalemia)
- Assessed the efficacy and safety of patiromer in 243 patients with HF with high levels of serum potassium.
- The recurrence of hyperkalemia during this period was significantly higher in the placebo group than in the patiromer group (60% versus 15%, P <0.001)
- The most common ADRs – constipation à It is under Phase 3 trial
Relaxin
- A naturally occurring hormone that is produced by the corpus luteum and placenta in pregnancy
- Mechanism of action à Relaxin interacts with a G protein-coupled receptor, leading to increased cGMP. As a result, nitric oxide production is increased by the increased activity of inducible nitric oxide synthase (iNOS)
- Its potent vasodilator properties as well as its ability to increase renal perfusion, relaxin became of interest as a potential therapy for acute HF
Vasodilators – Serelaxin
- Recombinant human relaxin- 2
- leads to vasodilation by increasing production of nitric oxide and upregulating the vascular endothelial B receptor and vascular endothelial growth factor.
- It ultimately leads to decreased systemic vascular resistance, increased cardiac output, and increased renal perfusion.
- Fails to meet primary endpoints in phase 3 RELAX-AHF-2 trial
Ularitide
- It is produced mainly by distal renal tubule cells and is secreted into urine and is involved in renal sodium handling
- MOA : It stimulates intracellular guanylyl cyclase & catalyses conversion of GTP to cGMP leading to venous & arterial dilation
- Natriuresis, diuresis, vasodilation, bronchodilation and inhibition of the renin–angiotensin–aldosterone system
- Trial of Ularitide Efficacy and Safety in Acute Heart Failure.
- A total of 2157 patients were enrolled to receive either the placebo or ularitide, a truncated form of the atrial natriuretic peptide, via a continuous 48- h intravenous infusion in addition to standard care.
- Even though ularitide produced a decrease in natriuretic peptides and blood pressure, no other primary end points such as mortality from cardiovascular causes were met
Empagliflozin
- In late 2015, the results of the Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients–
- Removing Excess Glucose trial, which treated patients with diabetes additionally with empagliflozin, an inhibitor of sodium glucose cotransporter 2, or the placebo, were published.
- After a median observation time of 3.1 years and 7020 enrolled patients,the empagliflozin group had a 38% relative risk reduction compared to the placebo.
- This trial and other promising data led to the initiation of several studies, for example, the EMPagliflozin outcomE tRial in Patients With chrOnic heaRt Failure With Reduced Ejection Fraction trial, which is expected to be completed in 2020 and which is evaluating the use of empagliflozin in patients with HFrEF.
Direct renin inhibition
- After the success of several agents that block the renin–angiotensin–aldosterone system (RAAS), direct renin inhibition with aliskiren in combination or as single therapy for heart failure was studied.
- However, in the ATMOSPHERE trial (Aliskiren Trial to Minimize Outcomesin Patients with Heart failure)
- the use of aliskiren in combination with enalapril was not associated with a decrease in heart failure hospitalization or cardiovascular death.
- Moreover, worse outcomes were seen with combination therapy, including hypotension, hyperkalemia, and renal dysfunction.
- Aliskiren alone as replacement for enalapril did not meet the specified noninferiority criteria and is, therefore, not recommended as a replacement for angiotensin-converting enzyme inhibitors (ACEI) in heart failure patients
Levosimendan
- works on cardiac contractile elements by increasing calcium sensitivity and thus, increasing contractility.
- It also causes vasodilation by blocking adenosine triphosphate-dependent potassium channels.
- Levosimendan is unique as its effects are not attenuated by concomitant beta blockade and has a long half-life.
- In the Randomized Multicenter Evaluation of Intravenous Levosimendan Efficacy (REVIVE II) study, levosimendan was associated with modest symptom relief but was also associated with a signal for possible harm with more frequent hypotension and cardiac arrhythmias
- The role of prophylactic levosimendan in patients with LV dysfunction undergoing cardiac surgery did not provide any clinical benefit as compared with placebo