Essential Drugs
- 1st WHO model list of essential drugs- 1977 contained 208 medicines. – the selection and procurement, at reasonable cost, of essential drugs of established quality corresponding to their national health needs”
- It stated that essential drugs were “of utmost importance, basic, indispensable and necessary for the health and needs of the population”.
- Revised every 2 years, List amended in November 2015
- 2007, a separate list for children up to 12 years
- Current version- 19th WHO Essential Medicines List
– 5th WHO Essential Medicines List for children
- Both updated in April 2015(36 added to adult and 16 to children list)
- Contents not mandatory -indicative list
- Provide guidance for 4 levels involved in selecting medicines:
- registration
- development of national lists
- development of lists in the hospital and medical environment
- medical prescription
Description of essential medicines – (Expert Committee Report, April 2002)
- Definition. Essential medicines are those that satisfy the priority health care needs of the population.
- Selection criteria: public health relevance, evidence on efficacy and safety, and comparative cost-effectiveness.
- Purpose: intended to be available within the context of functioning health systems at all times in adequate amounts, in the appropriate dosage forms, with assured quality & adequate information, and at a price the individual and the community can afford.
New procedures in 2002
- Term “essential medicines” instead of “essential drugs”
- ATC (Anatomical Therapeutic Chemical) classification of drugs
- More transparent process, systematic analysis of the evidence
- Full involvement of different WHO departments
- Absolute cost of a medicine not be a reason to exclude it from the Model List if it meets the stated selection criteria
- Cost-effectiveness comparisons made among medicines within the same therapeutic group e.g. for identifying the most cost-effective medicine treatment to prevent mother-to-child transmission of HIV
- Interested parties could comment on the application and its review to the Expert Committee
- Which medicines regarded as essential- national responsibility
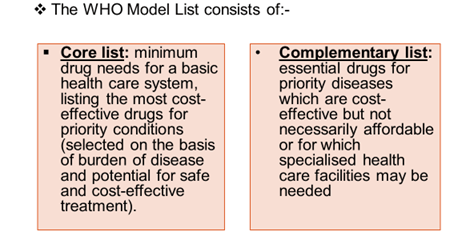
Priority conditions are selected based on current and estimated future public health relevance, and potential for safe and cost‐ effective treatment.
Importance of the who list
- Forms the basis of national drugs policy
- Procurement and supply of medicines in the public sector, schemes that reimburse medicine costs, medicine donations, and local medicine production
- International organizations, including UNICEF, UNHCR and UNFPA, NGOs and international non-profit supply agencies, adopt the essential medicines concept & base their medicine supply system mainly on the Model List.
- Powerful tool to promote health equity
Interagency list of essential medicines for reproductive health
Poor reproductive health accounts for about one-third of the total burden of disease among women of reproductive age and nearly one fifth of the disease burden in the general population like std, unplanned pregnancies
- 2006, WHO and UNFPA published the Interagency List of Essential Medicines for Reproductive Health- subset of the 14th Model List
- Contained 148 medicines, revised in 2009, 2011
- Only list devoted to products in a specific field of public health
- Key tool to:
- guide country decisions regarding reproductive health essential medicines
- guide international bulk procurement and support a core list
National List of Essential Medicine – NLEM
- In 1978, the World Health Assembly passed a resolution urging the Member States to establish national lists of essential medicines and adequate procurement systems.
- By MOHFW, GOI, 1st list 1996- 279 medicines
- Revision in 2003, 2011, 2015
- The NLEM 2015 has been prepared adhering to the basic principles of Efficacy, Safety, Cost-Effectiveness; consideration of diseases as public health problems in India. The list could be called as a Best-Fit List.
- NLEM 2015- 376 medicines.
- Coronary stents included in NLEM- to increase affordability
- Medicines used in dementia and neonatal care added, Dementia- donepezil, neonatal- surfactant,
- Any medicine/ vaccine, under a National Health Programme will be deemed to have been included in NLEM.
- Vaccines/ immunoglobulins/ sera in NLEM, irrespective of variation in source, composition and strength, all the products of the same vaccines/ immunoglobulins/ sera approved by the licensing authority are considered included.
- Essentiality considered in terms dosage form and strength also.
- Oral solid dosage form (tablet/capsule)- two aspects considered
- 1) dosage form that is commonly available
- 2) dosage form that is mentioned in Indian Pharmacopoeia
- Formulations developed through innovation/ novel drug delivery systems considered as included only if specified in the list against any medicine.- lipid/liposomal= amphotericin B, SR=metoprolol, isosorbide mononitrate
- Active moieties, without mentioning the salt – Active moities= diclofenac sodium and potassium
- Active moiety available as different isomers/ analogues/ derivatives, considered as separate entities, and inclusion of one does not imply inclusion of all isomers/ analogues/ derivatives.
- Fixed Dose Combinations (FDCs) not included unless, combination has unequivocally proven advantage over individual ingredients administered separately, in terms of increasing efficacy, reducing adverse effects and/or improving compliance.
- Over 50 representations- pharmaceutical industries, NGOs, associations/bodies, ministries– their viewpoints
Levels of healthcare in NLEM
- Medicines in NLEM are listed with reference to the levels of healthcare,
i.e., Primary (P), Secondary (S) and Tertiary (T) as the treatment facilities, training, experience and availability of health care personnel differ at these levels.
- 209 medicine formulations- all levels of health care (P, S, T),
- 115 medicine formulations- secondary and tertiary levels (S, T)
- 79 medicine formulations- the tertiary level (T).
- Formulations of certain medicines are listed at different levels but as item, they are counted as one.
Inclusion criteria of medicine in NLEM 2015
- Medicine must be licensed and approved in the country by DCGI
- Useful in disease which is a public health problem in India
- Proven efficacy and safety profile based on valid scientific evidence
- Comparatively cost effective
- Aligned with current treatment guidelines for the disease
- Stable under storage conditions in India
- Total treatment price considered, not the unit price of medicine
Criteria for deletion of medicine in NLEM 2015
- Medicine banned in India
- Reports of concerns on the safety profile
- Medicine with better efficacy or safety profile and better cost effectiveness is available
- Disease burden for which medicine is indicated is no longer a national health concern
- In case of antimicrobials, resistance pattern has rendered the medicine ineffective
Purpose of NLEM
- Guide safe and effective treatment of priority disease conditions of a population
- Promote the rational use of medicines
- Optimize the available health resources of a country
- Guiding document for:-
- State governments to prepare their list of essential medicines
- Procurement and supply of medicines in the public sector
- Reimbursement of cost of medicines by organizations to its employees
- Reimbursement by insurance companies
- Identifying the ‘MUST KNOW’ domain for the teaching and training of health care professionals
State lists
- Tamil Nadu- first state to develop the EML- 1994
- Delhi is the initiator in developing a comprehensive policy (Delhi State Drug Policy) in 1994
- For state government health facilities -standard guidelines (STGs). The armed forces medical college (AFMC) expanded STGs.
- Current- CPA(Central Procurement Agency) Essential Medicines List 2016
- Gujarat Essential Drug List 2016-17:
- Primary Health Care(PHC, Subcentres and others)-244
- Secondary Health Care(CHC and TB Hospitals)-369
- Tertiary Health Care(Districts, Subdistricts and Medical colleges)-556
- Chattisgarh, Rajasthan, Bihar, Orrisa, Meghalaya, Uttarakhand among states with EML.
- No Essential drug list for Maharashtra
Price cap in essential medicines
- GOI promulgated the NPPP (National Pharmaceuticals Pricing Policy), 2012- all medicines with specified dosage and strength in NLEM under price control
- Accordingly, DPCO (Drug Price Control Order), 2013 issued by Department of Pharmaceuticals under Ministry of Chemicals and Fertilizers for fixing the ceiling price of medicines included in NLEM
- Maximum retail price (MRP) <= ceiling price (plus local taxes as applicable) as notified by the Government for respective medicines.
- As per DPCO, 2013 ceiling prices- average retail price of the medicine, produced by all those companies engaged in its production with a market share of ≥ 1% of the total market turnover, and adding 16% margin to retailer
EDL and rational use of medicines
- The rational use of drugs requires that patients receive medications appropriate to their clinical needs, in doses that meet their own individual requirements for an adequate period of time, and at the lowest cost to them and their community.
- Selection of essential medicines- improvement of the quality of health care- appropriate use
- An analysis of prescription patterns between 1997 and 2003 -the average number of medicines prescribed per clinic visit decreased, as did the percentage of all medicines prescribed that were antibiotics and injections; the percentage of all medicines included in the essential medicines list being prescribed increased by about 5%.
