Thiazolidinediones (glitazones: rosiglitazone and pioglitazone)/ “insulin sensitizers”
- lower your blood sugar by increasing the muscle, fat and liver’s sensitivity to insulin.
- referred to as “insulin sensitizers” and also are blood sugar normalizing or euglycemics
- thiazolidinediones decrease insulin resistance.
- TZDs take a while to begin working (several weeks)
In 2010, the European Medicines Agency suspended sales of rosiglitazone, and in June 2011, the French and German Medicines Agencies also suspended the use of pioglitazone, owing to concerns that the overall risks of rosiglitazone and pioglitazone exceed their benefits.
A third, troglitazone, was the first drug in this class to be marketed but was removed from the market in both the United States and United Kingdom because it caused liver dysfunction and, in some patients, liver failure.
MECHANISM OF ACTION
Insulin sensitivity —
The thiazolidinediones increase insulin sensitivity by acting on adipose, muscle, and liver to increase glucose utilization and decrease glucose production
They bind to and activate one or more peroxisome proliferator-activated receptors (PPARs), which regulate gene expression in response to ligand binding.
PPAR-gamma is found predominantly in adipose tissue, pancreatic beta cells, vascular endothelium, macrophages, and the central nervous system (CNS). PPAR-alpha is expressed mostly in liver, heart, skeletal muscle, and vascular walls. The various thiazolidinediones have differential effects on PPAR-gamma and PPAR-alpha.
Rosiglitazone is a pure PPAR-gamma agonist, while
pioglitazone also exerts some PPAR-alpha effects (similar to fibrates). This may account for the different effects that pioglitazone and rosiglitazone have on lipids and cardiovascular ischemic outcomes
The concentration of PPAR-gamma is increased in the skeletal muscle of obese and diabetic patients; the raised concentration correlates well with the serum insulin concentration. It appears that the thiazolidinediones improve insulin responsiveness in skeletal muscle by facilitating glucose transport activity, thereby increasing rates of muscle glycogen synthesis and glucose oxidation.
In human adipocytes, rosiglitazone treatment has been reported to increase expression of genes involved in promoting lipid storage and decrease expression of genes associated with inflammation, such as interleukin-6 (IL-6). Thus, the insulin-sensitizing effect of thiazolidinediones may be related in part to regulation of adipose tissue production of adipokines via PPAR-gamma activation.
In addition, CNS PPAR-gamma activation mediates weight gain by promoting increased feeding. This finding accounts, in part, for the weight gain associated with thiazolidinedione treatment.
CLINICAL USE
Choice of agent —
If a thiazolidinedione is to be used, pioglitazone is preferred.
The use of rosiglitazone is not recommended, In 2010, the US Food and Drug Administration (FDA) imposed marked restrictions
because of the greater concern about its atherogenic lipid profiles and a potential increased risk for cardiovascular events (increased risk of acute myocardial infarction (MI)
These restrictions were largely removed by the FDA in 2013 after a reevaluation of the Rosiglitazone Evaluated for Cardiac Outcomes and Regulation of Glycaemia in Diabetes (RECORD) study, and the Risk Evaluation and Mitigation Strategy (REMS) (aimed at educating clinicians about the cardiovascular side effects of rosiglitazone) was eliminated in 2015. The absence of an adverse effect of rosiglitazone on cardiovascular risk, however, has not been excluded.
Potential indications
●Monotherapy –
Initial treatment of patients with type 2 diabetes mellitus includes education, with emphasis on lifestyle changes, including diet, exercise, and weight reduction when appropriate.
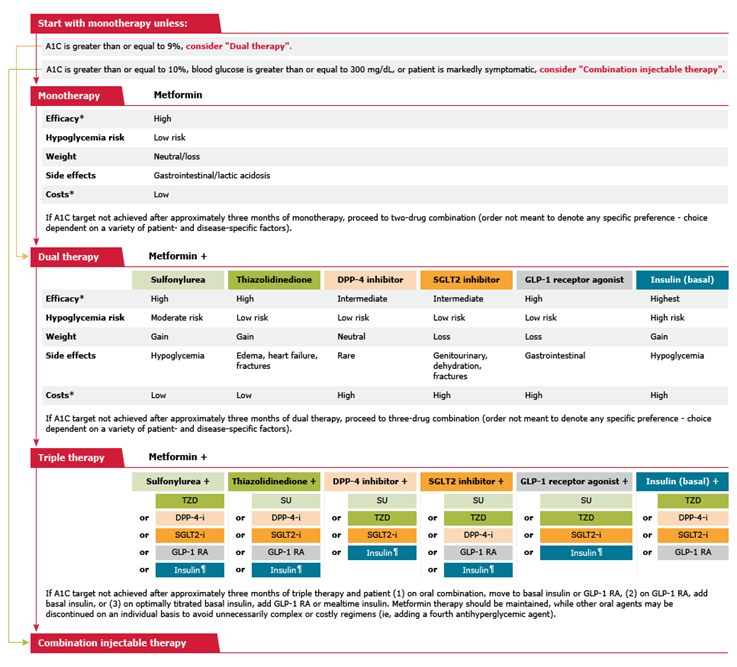
In the absence of contraindications, metformin is considered initial pharmacologic therapy for most patients with type 2 diabetes because of glycemic efficacy, absence of weight gain and hypoglycemia, general tolerability, and favorable cost.
Although thiazolidinediones are probably similar in efficacy to metformin as monotherapy, we do not generally choose thiazolidinediones over metformin for initial therapy of type 2 diabetes, due to concerns about adverse effects (weight gain, edema/heart failure, fractures). However, for patients with contraindications to metformin or sulfonylureas, pioglitazone is an option for initial therapy
●Combination therapy –
Although thiazolidinediones are effective for combination therapy in patients who have failed monotherapy with a sulfonylurea, metformin, or insulin and are included as an option in the American Diabetes Association (ADA) consensus algorithm
typically prefer combination with drugs other than thiazolidinediones because of adverse effects (weight gain, edema/heartfailure, fractures).
In certain clinical settings, however, such as especially high risk for hypoglycemia or intolerance of or contraindications to sulfonylureas, pioglitazone may be added.
Contraindications — Thiazolidinediones should not be used in patients with:
●Symptomatic heart failure
●New York Heart Association class III or IV heart failure
●Active bladder cancer or history of bladder cancer
●History of fracture or at high risk for fracture (eg, postmenopausal women with low bone mass)
●Active liver disease (liver enzymes >2.5 times above the upper reference limit)
●Type 1 diabetes
●Pregnancy
Dosing and monitoring —
Pioglitazone is usually initiated at a dose of 15 to 30 mg daily.
Rosiglitazone is usually initiated at a dose of 4 mg daily. If the glycemic response is inadequate after 8 to 12 weeks, the dose can be increased to a maximum of 8 mg daily (single daily dose or in divided doses twice daily).
●Glycemia – We obtain A1C at least twice yearly
●Heart failure – After initiation of pioglitazone or rosiglitazone, patients should be monitored for clinical signs of heart failure (eg, excessive rapid weight gain, dyspnea, edema).
●Liver enzymes – LFTs should be measured prior to initiating pioglitazone or rosiglitazone and periodically thereafter. Patient should be monitored clinically for symptoms that may indicate liver injury (eg, dark urine, jaundice, right upper abdominal discomfort) and the drug discontinued if liver enzymes are >3 times the upper reference limit.
The most common side effects are:
- Fluid retention
- Weight gain
- Anemia
- Increased LDL
- Congestive heart failure
- Increase risk of heart disease (rosiglitazone)
- Increased bone fractures in women
- Although there are no data linking rosiglitazone to bladder cancer, there is concern that pioglitazone use increases the risk of bladder cancer. The association is controversial, and different reports have given conflicting results. Pioglitazone should not be used in patients with active bladder cancer
CARDIOVASCULAR EFFECTS
The cardiovascular effects of thiazolidinediones may depend upon the specificity of peroxisome proliferator-activated receptor (PPAR) activation
Rosiglitazone (a pure PPAR-gamma agonist) and
pioglitazone (PPAR-gamma with some PPAR-alpha agonist activity) have different effects on lipid concentrations, with pioglitazone exerting a less unfavorable effect. Although both drugs have a similar effect on the incidence of heart failure (both increase risk), they appear to have disparate effects on ischemic outcomes
Rosiglitazone use appears to be associated with a higher risk of adverse cardiovascular events than pioglitazone (Rosiglitazone Evaluated for Cardiac Outcomes and Regulation of Glycaemia in Diabetes (RECORD) study)
Prospective Pioglitazone Clinical Trial in Macrovascular Events (PROactive) trial
Lipids — Rosiglitazone and pioglitazone have similar effects on glycemic control, but their effects on serum lipid concentrations are different. Most randomized trials found that pioglitazone produces a more favorable lipid profile
Investigational PPAR agonists — Muraglitazar, a dual peroxisome proliferator-activated receptor (PPAR)-alpha and gamma agonist, was associated with an excess incidence of adverse cardiovascular events, including MI, and is no longer under development.
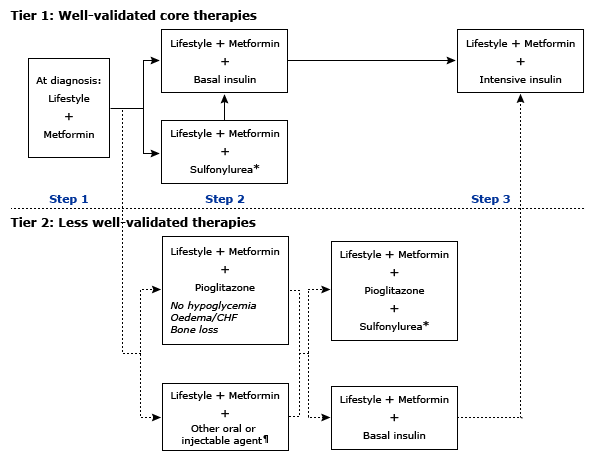
* Sulfonylureas other than glybenclamide (glyburide) or chlorpropamide.
¶ Alternative agents include: GLP-1 agonists, DPP-4 inhibitors, alpha-glucosidase inhibitors, SGLT2 inhibitors