Opioid receptors
- substances obtained from the crude extract of Papaver somniferum (poppy plant).
- Morphine is the prototype opioid and acts by agonistic activity on μ, k and d receptors.
- critical in the modulation of pain behaviour and anti-nociception
Types
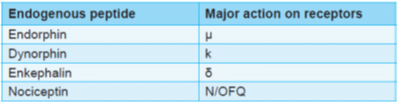
Recently a new endogenous peptide, nociceptin is isolated that acts on nociceptin/orphanin FQ (N/OFQ) or orphanin like receptors (ORL1)
Role of Endogenous Ligands:
Enkephalins – DOP
- CNS and Adrenal medulla
- Hypothalamus, Nucleus accumbens, Ventral tegmental area, Neocortex
- Pain Modulation, Memory and emotions, feeding, immune Response, control of gonadal functions
Endorphins – MOP
- Analgesia and neuroendocrine functions, Modulation of cardiovascular responses.
- Trigger: Hypoxia, acidosis, stress and inflammation
Dynorphins – KOP
- CNS & GIT.
- Modulation of stress, cognitive function and motor integration.
Opioid receptor transducer mechanisms
- all are GPCRs located mostly on prejunctional neurons
- Opioid receptor activation reduces intra cellular cAMP formation and opens K+ channels
- (mainly through µ and d receptors) or suppresses voltage gated N type Ca2+ channels (mainly k receptor
- result in neuronal hyperpolarization and reduced availability of intracellular Ca2+ → decreased neurotransmitter release by cerebral, spinal, and myenteric neurones (e.g. glutamate from primary nociceptive afferents
Pharmacokinetics
- Sufentanil is the most potent whereas meperidine (pethidine) and propoxyphene are the least potent opioids.
- Morphine is metabolized mainly to morphine-3-glucuronide (M3G) that has neuroexcitatory properties. Approximately 10% of morphine is metabolized to active product M6G. Renal failure can lead to accumulation of these metabolites and can result in seizures (due to M3G) or prolonged opioid action (due to M6G).
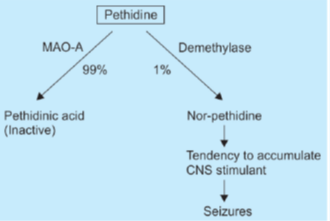
- Pethidine is metabolized mainly to meperidinic acid by MAO and very little is demethylated to norpethidine.
- Latter has seizure inducing and cumulative properties.
- Pethidine can result in seizures if used for prolonged periods, in patients with renal failure or those taking MAO inhibitors (due to accumulation of norpethidine).
Actions of pure opioids
Pure agonists include morphine, methadone, pethidine, levorphanol, codeine, hydrocodone,
oxycodone and propoxyphene.
1. CNS Actions
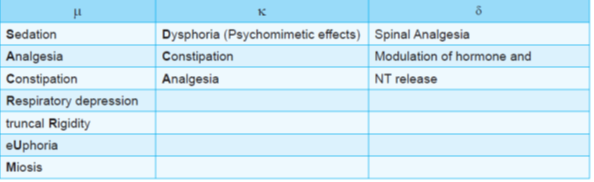
- Morphine produces spinal and supraspinal analgesia by acting on μ, κ and δ receptors.
- μ receptor opioids have dependence producing actions due to euphoric action.
- Κ receptors mediate psychomimetic effects (dysphoria).
- Tolerance develops to all actions of opioids except 3C (Constipation, convulsions and constriction of pupil)
- Opioids produce marked sedation but chances of sedation are less with pethidine and fentanyl.
- Opioids can produce respiratory depression and cough suppression.
- Miosis can occur with morphine use and pin point pupil is a valuable sign in diagnosis of opioid poisoning.
- Highly lipid soluble drugs like fentanyl, alfentanyl and sufentanil can result in truncal rigidity on rapid i.v. infusion.
- By stimulating CTZ, opioids can result in nausea and vomiting.
2. Peripheral Effects
- Opioids have no direct effect on heart except pethidine and pentazocine (that increase heart rate).
- Blood pressure may decrease due to depression of vasomotor system and release of histamine.
- There is a shift of blood from pulmonary to systemic circuit due to greater vasodilatation in the latter
- Constipation can result due to decreased motility and increased tone of GIT.
- Alvimopan is a peripheral opioid antagonist developed for paralytic ileus.
- Opioids increase intrabiliary pressure by constricting biliary smooth muscle. (C/I in biliary colic).
- may aggravate bronchoconstriction in asthmatics by releasing histamine. (C/I in asthmatics).
- Spinal or epidural administration of opioids may result in intense pruritus over lips and torso (due to histamine release).
Actions of Mixed Agonists-antagonists
Buprenorphine
- partial agonist at m receptor with k and d antagonistic property
- useful as an analgesic and as an alternative to methadone for the management of opioid withdrawal.
Nalbuphine, pentazocine and dezocine (K analgesic)
- k agonists and μ receptor antagonists.
- can produce psychomimetic effects with hallucinations, nightmares and anxiety.
- Pentazocine à weak μ antagonistic and more marked κ agonistic actions.
profile of action is similar to morphine; important differences are:
- Analgesia caused by pentazocine is primarily spinal (κ1) and has a different character than that caused by morphine.
- Parenterally 30 mg pentazocine = 10 mg morphine
- Sedation and respiratory depression is 1/3 to 1/2 of morphine at lower dose
- Tachycardia and rise in BP are produced at higher doses due to sympathetic stimulation
- Biliary spasm and constipation are less severe.
- Vomiting is less frequent
- Abuse liability is rated lower than morphine
Butorphanol
- predominant k agonist that produces equivalent analgesia but more sedation than morphine.
Clinical uses
- These are used as analgesic agents.
- Visceral, dull and constant pain is relieved more effectively than inflammatory pain.
- Opioids are however contraindicated in biliary colic.
- Morphine (i.v.) is useful in myocardial infarction as well as in acute pulmonary edema.
- Codeine, pholcodeine, dextromethorphan and noscapine are effective cough suppressants.
- Dextromethorphan is devoid of constipating action unlike other drugs in this group.
- Loperamide and diphenoxylate can be used for the treatment of non-infective diarrhea.
- Morphine is useful as a pre-anaesthetic medication whereas highly lipid soluble drugs (like fentanyl, alfentanil, sufentanil etc) are used as adjuncts to other anaesthetic agents.
- Pethidine is used to reduce shivering after anaesthesia [by its action on a2 receptor]
Routes of Administration
- Morphine can be administered by oral, rectal, i.v., i.m., intrathecal or epidural routes.
- Fentanyl can be applied as transdermal patch or can be administered by buccal transmucosal route.
- Butorphanol is the only opioid available in nasal formulation.
Adverse Effects and Toxicity
- Respiratory depression, nausea, vomiting, constipation, urinary retention, itching and dysphoria are important adverse effects of opioids.
- Tolerance develops to most of the actions of opioids except miosis, constipation and convulsions.
- Opioids are highly addictive substances and can lead to development of psychological as well as physical dependence.
- Sudden discontinuation à withdrawal syndrome
- rhinorrhoea, lacrimation, yawning, chills, mydriasis, vomiting, diarrhea and anxiety.
Contraindications and Precautions
- Morphine à absolutely contraindicated in head injury because it increases intracranial tension by causing retention of CO2 (due to respiratory depression).
- also interferes with the assessment of neurological function by masking the important pupillary signs (causes miosis).
- should be used cautiously in patients with pulmonary, hepatic or renal dysfunction.
- Use of opioids in infants and elderly also require caution.
- Prolonged use of opioids in pregnancy may lead to in-utero physical dependence of fetus and severe withdrawal symptoms may be precipitated after birth.
Important Points about Specific Agents
Methadone
- long acting opioid analgesic that can be administered by oral, i.v., s.c. and rectal routes.
- Apart from potent agonistic actions at μ receptors, it also blocks NMDA receptors and reuptake of monoamines.
- relieve neuropathic and cancer pain that are not controlled with morphine.
- Due to its long t1/2, development of dependence and tolerance is very slow, making it useful for the treatment of opioid abuse.
- It is also useful for opioid rotation therapy.
Pethidine and pentazocine
- possess anticholinergic activity (can result in tachycardia).
- These drugs are therefore C/I in MI.
- Because of anticholinergic properties, these are relatively safer in biliary colic as compared to other agents.
- Accumulation of active metabolite of pethidine (norpethidine) can produce seizures.
Propoxyphene
- least potent and least efficacious analgesic agent.
- Diphenoxylate and its active metabolite difenoxin, as well as loperamide are useful for diarrhea.
- Buprenorphine dissociates slowly from μ receptors and is thus resistant to naloxone reversal.
Tramadol
- weak m receptor agonist. It also inhibits reuptake of NA and 5-HT.
- These effects are responsible for its analgesic action, which can be abolished by 5-HT3 antagonists like ondansetron.
- analgesic action is only partially reversed by the opioid antagonist naloxone.
- At high doses, it can lead to seizures.
- Tramadol causes less respiratory depression, sedation, constipation, urinary retention and rise in intrabiliary pressure than morphine.
- Tramadol should not be given to patients taking SSRI therapy because of risk of ‘serotonin syndrome
Tapentadol
- new analgesic drug with μ-receptor agonistic action and NA reuptake inhibiting action.
Opioid Antagonists
Naloxone, naltrexone and nalmefene are potent m receptor antagonists with significant
blocking action at k and d receptors also.
Alvimopan and methylnaltrexone are peripheral opioid antagonists.
- Naloxone is given parenterally (ineffective orally) and is a very short acting drug.
- Nalmefene is also given parenterally but has a longer half life.
- Naltrexone is long acting orally effective opioid antagonist.
Actions
- have no action in the absence of agonists but promptly reverses the opioid effects when administered i.v.
Uses
- Naloxone is the drug of choice for acute opioid poisoning but it has to be repeated frequently.
- Naltrexone is used as a maintenance drug for opioid poisoning.
- It is also used to prevent relapse after opioid de-addition.
- It is also used to decrease craving in chronic alcoholics.
- Naltrexone plus bupropion has recently been approved for treatment of obesity
- Naloxone is also used in neonatal resuscitation to reverse the effects of opioids (if used during labour).
- However, it should not be used for this purpose if mother is dependent on opioids. (Baby is also dependent in utero and naloxone can precipitate withdrawal).
- Naloxone is being added to opioids meant for oral use to minimize their addictive potential.
- If the patient takes the combination orally, only opiod is absorbed not naloxone. Thus, it will produce the desired action. However, if the person takes it by i.v. route for addiction, naloxone also reaches the blood and stops euphoria.
- Methylnaltrexone and alvimopan are peripheral opioid antagonists indicated for opioid-induced constipation.
- Naloxegol is a new drug recently approved for same indication
Opiod De-addiction
- Chronic intake à physical and psychological dependence. I
- Suddenly stopped à withdrawal symptoms
- For de-addiction of opioids (or any addictive drug), first aim is to stop the further use of the drug by the patient followed by maintainance of de-addiction (i.e., to prevent relapse).
- If addiction is of short duration and with small doses of addictive drug, sudden stoppage of drug therapy can be attempted and the mild withdrawal symptoms can be treated with b-blockers or clonidine (or lofexidine).
- If addiction is of long duration or with large dose of opioids, sudden withdrawal of the offending drug may be dangerous (due to severe withdrawal symptoms).
- In such patients, the addictive drug is replaced by equivalent dose of methadone (known as methadone maintenance).
- It prevents withdrawal symptoms by stimulating opioid receptors but is much less addictive. The dose of methadone is then gradually decreased and finally stopped.
- To prevent relapse after de-addiction, naltrexone is used.
- Naltrexone prevents euphoric action by blocking μ receptors. If the person again takes opioids (after deaddiction), there will be no euphoria and the person’s resolution to quit addiction will be strengthened.
Note:
• b-blockers and clonidine treat withdrawal symptoms.
• Methadone prevents withdrawal symptoms. • Naltrexone is used to prevent relapse.
• Methadone is used as maintenance therapy in opioid dependence whereas naltrexone is used as
maintenance therapy in opioid poisoning.
